Where to Now, Phospho-Tau?
Quick Links
Plasma phospho-tau took the Alzheimer’s field by storm in 2020. Paper after paper described how p-tau181, p-tau217, and p-tau231 specifically and sensitively picked up the disease, especially in its presymptomatic phase. So: Are we ready for a simple phospho-tau blood test for AD? At this year’s virtual ADPD meeting last month, the collective answer was: Not quite.
- Plasma p-tau isoforms may not work as stand-alone markers for AD.
- Algorithms that consider additional factors look promising.
- These need to be validated in the general population.
- P-tau might simplify clinical trial selection and monitoring.
That said, a clearer picture did emerge of how these biomarkers may be used clinically before too long. For older people without cognitive problems, p-tau appears insufficient to identify who might have presymptomatic AD. Even for people who have been diagnosed with some form of cognitive impairment, p-tau markers cannot stand alone. Instead, researchers are building and testing out algorithms based on several different biomarkers and other risk factors, such as age and APOE genotype.
This approach assumes that the impressive correlations between plasma p-tau isoforms and AD pathology seen in selected cohorts hold true in the general population. “We are just really at the beginning of this field,” said Michael Weiner, University of California, San Francisco, in a session on biofluid markers. “We need bigger, more epidemiologically sampled populations that better represent the community than do clinical trial populations. We need a lot more studies, and we need more data to compare the different tests,” said Weiner. He stressed that most of the studies to date have come from “samples of convenience,” i.e., highly educated Caucasians.
P-tau, You’ll Never Walk Alone?
In various memory center cohorts, p-tau181, p-tau217, and p-tau231 distinguish Alzheimer’s disease from controls with such high accuracy that some have suggested these markers are good enough to become AD diagnostics by themselves. Not all are on board with this idea, even based on the cohorts that exist today. At ADPD, Cliff Jack, Mayo Clinic, Rochester, Minnesota, argued that these markers are not quite up to snuff. “For this notion of a stand-alone, one point of caution is that these markers should differentiate amyloid-positive from -negative in individuals at all points along the disease spectrum. Studies cast doubt on this,” he said.
One such study comes from ADNI. Weiner, ADNI's principal investigator, reported that among people in that cohort who are cognitively impaired, plasma p-tau181 distinguishes amyloid-positives with a moderate AUC of around 0.67, much lower than the AUCs of 0.77 to 0.91 reported in some memory clinic cohorts (Janelidze et al., 2020; Karikari et al., 2020; Thijssen et al., 2020).
This new data comes from a study led by Duygu Tosun at UCSF and was reported in February in the journal Brain Communications.
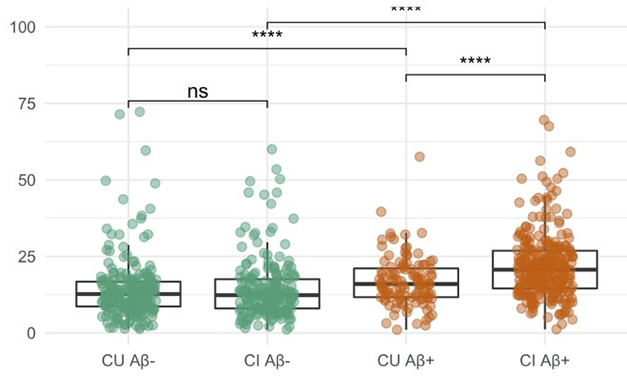
Much Overlap. In ADNI, people with brain amyloid (brown) have only moderately higher plasma p-tau181 than people without (green). The difference is smaller among the cognitively unimpaired (CU) than the impaired (CI). Group differences are highly statistically significant but individual levels show much overlap. [Courtesy Tosun et al., 2021.]
Tosun found that among cognitively unimpaired ADNI participants, plasma p-tau181 distinguished 109 amyloid-positives from 224 -negatives with even less accuracy—AUC of 0.5. That does not bode well for a stand-alone marker for the general population.
Likewise, in a small cohort at Washington University, researchers led by Nicolas Barthélemy and Randall Bateman found that plasma p-tau181 distinguished 20 cognitively normal amyloid-positive people from 31 amyloid-negative people with an AUC of only 0.67 (Barthélemy et al., 2020).
In the larger Mayo Clinic Study of Aging, a prospective population-based cohort, Michelle Mielke at Mayo in Rochester, Minnesota, had previously found that this tau marker identified 72 amyloid-positives among 172 cognitively unimpaired with an AUC of 0.7. At ADPD, Mielke reported that in a larger sample of 892 cognitively unimpaired people, p-tau181 performed only slightly better, with an AUC of 0.78.
Is p-tau181 the wrong species? While the few studies that have analyzed p-tau217 and p-tau231 thus far suggest that these may prove more accurate, they still may not be accurate enough, Mielke said. She reported that p-tau217 identifies amyloid-positives among the MCSA cognitively unimpaired participants with an AUC of 0.83. In his much smaller sample, Barthélemy calculated 0.86 for p-tau217.
After all the excitement for blood tests, what explains the different accuracies now being reported in additional cohorts for p-tau181? Mielke has begun to study the effects of various health parameters on plasma levels of p-tau181 and p-tau217. In her ADPD presentation, she reported that among the cognitively unimpaired, a higher score on the Charlson comorbidity index correlated with higher levels of both tau isoforms in the plasma. Chronic kidney disease by itself had an even greater effect. Mielke thinks this is because of a failure to clear the isoforms from the blood. On the other hand, high body mass index associated with lower levels of the two markers, possibly because there is more blood to dilute them.
Even among memory clinic populations, i.e., those “convenience samples,” plasma tau markers may be insufficient by themselves to serve as a diagnostic. Christopher Clark, University of Zurich, reported p-tau181 and neurofilament light (NfL) data from 221 people recruited from a memory clinic there. Among the 91 who were cognitively normal, p-tau181 could not identify those who had been diagnosed with AD based on CSF markers.
Enter Algorithms
What about combining p-tau with other markers? Clark reported that adding plasma p-tau181 to a reference AD prediction model that marries age, sex, years of education, and ApoE increased the model's AUC from 0.82 to 0.86. Most other groups have come to similar conclusions. Mielke reported that adding the age and ApoE4 genotype of cognitively unimpaired people increased the accuracy of plasma p-tau181 to identify amyloid-positives among them from AUC 0.78 to 0.84. For p-tau217, it upped the AUC to 0.86. By the same token, Tosun found that adding clinical information for the cognitively unimpaired, including age, sex, years of education, APO4 status, and global cognitive test scores, boosted p-tau181's AUC from 0.55 to 0.80.

Individualized Prediction. An online research tool allows users to test how various parameters, such as age, cognition, or plasma p-tau, increase a person's four-year risk of Alzheimer’s. [Courtesy of Oskar Hansson].
Researchers in Oskar Hansson’s group in Lund University, Sweden, are testing different plasma tau-based prognostic algorithms to see which one best predicts AD over a four-year period. They previously reported that in Sweden's BioFinder cohort, baseline plasma p-tau217 in people with mild cognitive impairment predicted deterioration to dementia with an AUC of about 0.88. In ADNI, plasma p-tau181 performed less well, but when the scientists added baseline NfL into the mix, the results were on par with BioFinder (Nov 2020 news on Cullen et al., 2020). This data led Niklas Mattsson-Carlgren at Lund to develop an online research tool based on age, sex, cognitive score, and plasma or CSF level of p-tau181 and NfL. It predicts deterioration to dementia over four years.
But what about people who do not already have cognitive impairment? In their ADPD presentations, Hansson and Sebastian Palmqvist, also from Lund, described how they are building algorithms from a broader range of measures that are sensitive in the preclinical phase of AD. They include plasma markers, scores in different cognitive domains, structural MRI, and demographics. They started with plasma p-tau217 and then added variables such as age, sex, APOE genotype, cortical thickness, memory, executive function, verbal recall, and plasma NfL until hitting upon the most predictive combination. The best model had six ingredients: plasma p-tau217, plasma NfL, APOE genotype, cortical thickness, delayed recall for memory, and Trail Making Test B for executive function. This pegged conversion to AD within two, four, or six years with AUCs of 0.91, 0.92, and 0.94, respectively. This trajectory indicates that this algorithm will predict AD even better over a longer time frame.
An algorithm that relied on only four ingredients—plasma p-tau217, APOE, memory, and executive function—performed nearly as well, with AUCs only 0.01 lower. Interestingly, this algorithm still did a much better job than did dementia clinicians at baseline, whose own AUC for AD in four years was 0.72. “Clearly, this algorithm is very powerful at predicting AD dementia,” said Hansson.
Next, the researchers tested their algorithms in the ADNI dataset. Here, the best model was slightly different. It included sex and years of education, plasma p-tau18 but not plasma NfL. (In ADNI, p-tau217 was not measured). Still, the AUC for a four-year prediction reached 0.91, and the four-parameter model worked almost as well, again yielding AUCs only 0.01 lower.
Finding Cutoffs
The different plasma p-tau measures—217 in one cohort and 181 in the other—presented Palmqvist with the quandary of how to directly compare algorithms for the BioFinder and the ADNI datasets. To solve it, he treated each p-tau marker as either positive or negative based on a cutoff, rather than treating each as the continuous variable that it truly is. While this reduced the accuracy, yielding AUCs of 0.89 and 0.86 for BioFinder and ADNI, respectively, now the two algorithms were directly comparable. Anyone can try out these research algorithms using an online tool.
For real-world deployment of any plasma test, scientists will have to set cutoff values for plasma p-tau species that indicate a person's risk for dementia, much like cholesterol values do for cardiovascular disease and insulin for diabetes. Setting cutoffs is yet another task of applied science that commands no glory but is oh-so-important for any test to work robustly at your local doctor's office. AD researchers have begun testing these waters.
Clark determined that a cutoff of 9.6 pg/mL for p-tau181 improved the prediction of an algorithm based on age, sex, years of education and APOE, bumping up its AUC from 0.82 to 0.87.
For her part, Mielke has tested various cutoff values using data from a clinical trial population. Plasma p-tau181 and p-tau217 values of 1.56 pg/mL and 0.25 pg/mL, respectively, or greater, predicted amyloid-positivity with specificities of 92 percent and 96 percent, respectively. Alas, sensitivities were low, around 50 percent, meaning the algorithm missed half of the people who did have brain amyloid. Reducing the cut points to 1.31 and 0.23 pg/ml for p-tau181 and p-tau217, respectively, improved the sensitivities by 15 percent and 7 percent, without compromising specificity much.
Still, these numbers are meh. “A lot of work is going to be needed to identify the best cut points for screening, or perhaps diagnostic purposes, and that can be replicated across cohorts,” Mielke said.
Case in point: the sixfold difference in cutoff values that Clark and Mielke settled on. Clark told Alzforum that he opted for a value that would maximize his ability to predict “cerebral AD” in cognitively impaired subjects. By cerebral AD, he means a CSF p-tau181/Aβ1-42 ratio of greater than 0.0779. “One must remember that the cut-offs are dependent on the model in which they are used, including confounders, but also on the cohort.” Clark believes it is difficult to harmonize cut-offs since many parameters differ between studies. “Nonetheless it is encouraging that defining these is plausible, because it opens up the possibility of efficient and reliable diagnosis and risk assessment in the clinic,” he said.
Mielke cautioned about co-morbidities, such as BMI and kidney disease, that influence plasma p-tau levels. “This will be important when interpreting levels for clinical patients, especially in the general population, and also in the development of reference ranges,” she said. “Additionally, as studies examine levels across race and ethnicity, it is very important to consider these variables in the interpretation of any notable differences that could be due to comorbidities and health disparities, and therefore not to overinterpret results as solely due to race or ethnicity.”
All told, researchers agree that much work remains to be done before these exciting markers are ready for prime time. Besides cut-offs, they are beginning to address questions such as which isoform, assay, and platform might be best, how reproducible those assays are, and how they might work in different populations and for what purpose. In one bit of good news, Mielke reported that over three consecutive tests 15 months apart, p-tau181 and p-tau217 levels were within 92 and 95 percent agreement in the same person, respectively, suggesting that at least intra-individual variability is low. Likewise, in his presentation, Kaj Blennow, University of Gothenburg, reported that the coefficient of variation for plasma p-tau181 over 24 months was about 7 percent in cognitively unimpaired groups, a bit higher in those with mild cognitive impairment, and 12 percent in people with AD. This, too, is far less than what was seen with, for example, CSF Aβ42 or total tau tests early in these markers' “careers.” “This low variability suggests that p-tau181 could be used to monitor drug effects on tau pathology in clinical trials of this duration,” he said.
Hansson thinks so, too. He showed data indicating that people whose plasma p-tau217 rises more steeply over six years decline faster on cognitive tests over the same period of time, suggesting the marker could be a good readout of disease progression.
Along those lines, Hansson thinks these markers are attractive for trials to use to select participants whose likely rate of progression can be estimated better than in years past. This may well happen before their rollout in broad clinical practice. His group reported that among people in BioFinder who were deemed cognitively normal, performance on the PACC declined much faster in those who tested positive for plasma p-tau217 as per the research group's internal cut off.
As has been done before with new biomarkers, Hansson calculated that a trial's sample size could be shrunk by 70 percent by prescreening candidate participants with a combination of plasma Aβ42/40, p-tau217, and NfL. The PACC was designed to detect subtle changes in cognition among people who still appear normal on standard cognitive batteries such as the ADAS-cog, which is most sensitive at a later, more clearly symptomatic stage of AD (Jun 2014 news).
Will plasma p-tau replace brain imaging? This remains to be seen. Researchers are indeed toying with this idea not just for tau, but for all three biomarker criteria in AD—amyloid, tangles, and neurodegeneration. “I think most people would agree that it’s pretty simple,” said Jack. “If plasma is ultimately as accurate as CSF/imaging, then plasma will supplant imaging biomarkers. But if plasma turns out not to be as accurate as imaging, then it is reasonable to expect that plasma will be used for screening, and CSF and imaging will be used for people who pass certain screening criteria.”
Hansson was optimistic on this score. “I think within five years we will have these assays set up on fully automated platforms, and we will be using them in specialized clinics to replace CSF and PET analysis,” he said. He cautioned that appropriate use criteria have to be defined. He is even optimistic that these tests will be used in primary care settings. “But we need to do a lot more studies in those populations,” he agreed.—Tom Fagan
References
News Citations
- Plasma Aβ Test Wins Approval—Are p-Tau Tests Far Behind?
- Test Battery Picks Up Cognitive Decline in Normal Populations
Paper Citations
- Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, Chai X, Proctor NK, Eichenlaub U, Zetterberg H, Blennow K, Reiman EM, Stomrud E, Dage JL, Hansson O. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020 Mar;26(3):379-386. Epub 2020 Mar 2 PubMed.
- Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, Chamoun M, Savard M, Kang MS, Therriault J, Schöll M, Massarweh G, Soucy JP, Höglund K, Brinkmalm G, Mattsson N, Palmqvist S, Gauthier S, Stomrud E, Zetterberg H, Hansson O, Rosa-Neto P, Blennow K. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020 May;19(5):422-433. PubMed.
- Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, Bourakova V, Cobigo Y, Heuer H, Spina S, VandeVrede L, Chai X, Proctor NK, Airey DC, Shcherbinin S, Duggan Evans C, Sims JR, Zetterberg H, Blennow K, Karydas AM, Teunissen CE, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Rabinovici GD, Dage JL, Rojas JC, Boxer AL, Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020 Mar;26(3):387-397. Epub 2020 Mar 2 PubMed.
- Barthélemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer's disease. J Exp Med. 2020 Nov 2;217(11) PubMed.
- Cullen NC, Leuzy A, Palmqvist S, Janelidze S, Stomrud E, Pesini P, Sarasa L, Allue JA, Proctor NK, Zetterberg H, Dage JL, Blennow K, Mattsson-Carlgren N, Hansson O. Plasma amyloid, phosphorylated tau, and neurofilament light for individualized risk prediction in mild cognitive impairment. medRxiv July 24, 2020. MedRxiv.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Tosun D, Veitch D, Aisen P, Jack CR Jr, Jagust WJ, Petersen RC, Saykin AJ, Bollinger J, Ovod V, Mawuenyega KG, Bateman RJ, Shaw LM, Trojanowski JQ, Blennow K, Zetterberg H, Weiner MW. Detection of β-amyloid positivity in Alzheimer's Disease Neuroimaging Initiative participants with demographics, cognition, MRI and plasma biomarkers. Brain Commun. 2021;3(2):fcab008. Epub 2021 Feb 2 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University
The data on blood tests for AD, both Aβ42/Aβ40 and pTau isoforms, are quite exciting to me as both a researcher and a clinician. Algorithms that incorporate individual-level factors (e.g., age, sex, APOE genotype, race) and multiple biomarkers are likely to be very powerful, and potentially much more predictive on an individual level than the traditional single-cut-off model.
I completely agree that we should test these biomarkers in large cohorts that are representative of the broader population. However, I would note that dementia specialists have been using CSF biomarkers and amyloid PET in select patients for well over a decade, without the benefit of massive datasets across many groups—and most dementia specialists and patients have found this information to be accurate and useful. …More
Given the rapid rate of development of blood-based biomarkers, do we really need to wait five years to incorporate them into clinical care? Is it reasonable to set the threshold for blood test accuracy so high, when the threshold for CSF biomarkers and amyloid PET was so much lower?
Very few patients with memory complaints (likely <5-10 percent) currently get AD biomarker testing because of cost and availability issues. A blood test could enable AD biomarker testing in the majority of patients with memory complaints. Would setting a very high threshold for an AD blood test mean that more patients are misdiagnosed, because for many patients, the real-world alternatives may be an AD blood test or no biomarker testing at all?
We should absolutely validate AD blood tests carefully to minimize inaccurate results, but waiting too long and setting too high a bar could also have negative consequences for all the patients who never get a biomarker test. Hopefully we can refine and test these assays expeditiously so that our patients can benefit sooner rather than later. But for the sake of our patients, we should also consider that sometimes the perfect is the enemy of the good. Or in this case, that having a very good AD blood test is a better alternative than the current status of most dementia patients, who have no AD biomarker testing at all.
Make a Comment
To make a comment you must login or register.