Tau PET Studies Agree—Tangles Follow Amyloid, Precede Atrophy
Quick Links
Part 1 of a two-part report. See Part 2.
As data from tau PET studies come rolling in, they paint a remarkably uniform picture of how tau accumulation fits into the pathophysiology of Alzheimer’s disease. Speakers at the Alzheimer’s Association International Conference 2016, held July 22-28 in Toronto, presented the latest findings from longitudinal and cross-sectional studies of aging and AD. Without exception, they reported that widespread tau pathology depends upon the presence of Aβ, and that the tau signal correlates closely with brain atrophy and declining cognition, even at preclinical disease stages. Researchers lauded the robustness of the findings, which held true across diverse study populations and for different tracers and methodologies. Together, the data strengthen the idea that tau PET could serve as a marker of AD progression and perhaps an outcome measure in trials, although this remains to be tested.
In all, the imaging data generated some of the biggest excitement at the conference. “I was really impressed by how the amount of tau imaging data has exploded in the last two years,” Christian Sorg of the Technical University of Munich told Alzforum. Not only do the data match previous postmortem findings about the patterns of neurofibrillary tangle spread, but tracers now allow researchers to track progression of tau pathology in people at preclinical disease stages and link it with other biomarkers, Sorg noted. Clifford Jack of the Mayo Clinic in Rochester, Minnesota, believes such data will open new frontiers for research. “Tau imaging is a transformational technology,” he said.
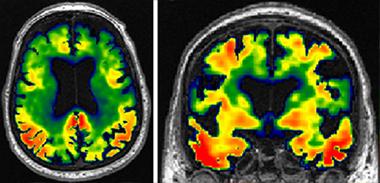
New View of Alzheimer’s.
Tau imaging reveals patterns of tangle accumulation in AD brains. [Courtesy of Clifford Jack.]
Amyloid Sets Off Tangles
Although tau is not the only cause of neurodegeneration (see Aug 2016 conference news), widespread tangles in the brain generally spell trouble. Initial tau PET studies had already suggested a close association between regional tau pathology and brain atrophy, in agreement with past autopsy studies (see Feb 2015 conference news). The extent of tangles, unlike amyloid plaques, also correlates with cognitive decline (see Nov 2013 conference news; Aug 2014 conference news; May 2016 news). Plaques play a key role as well, however, as numerous groups have now reported that tangles spread through the brain only when amyloid has accumulated (see Mar 2016 news; Jul 2016 news; Aug 2016 news).
At AAIC, researchers continued to build the case that amyloid plaques act as a trigger for tau pathology. Mark Mintun of Avid Radiopharmaceuticals, Philadelphia, a subsidiary of Eli Lilly, described a study of 57 cognitively normal and 96 cognitively impaired people, and 46 with possible or probable AD. Averaging around 70 years old, they all underwent PET scans with AV1451 (aka T807, aka flortaucipir) at baseline and again at nine and 18 months later. Those without evidence of amyloid on florbetapir PET had an average AV1451 standard uptake value ratio (SUVR) of 1.01 in the cortex, and this remained stable over the course of the study. Those with positive florbetapir scans bound more cortical AV1451 already at baseline (average SUVR of 1.32), and the higher their tau signal was, the likelier it was to rise further on follow-up visits (correlation of r = 0.74). Over time, tau deposits typically spread to new regions, rather than intensifying in existing ones, Mintun noted.
Similar data emerged from 474 participants in the Mayo Clinic Study of Aging, which studies 50- to 90-year-olds who are cognitively normal or have been diagnosed with mild cognitive impairment or dementia. People free of brain amyloid, regardless of cognitive status, had similarly low AV1451 uptake, said David Knopman of the Rochester Mayo Clinic. However, in cognitively normal people with amyloid, the tau tracer lit up the medial temporal and lateral temporal lobe. In cognitively impaired people with amyloid, signal intensity was even higher and extended to parietal regions as well. The researchers saw an even bigger tau signal in amyloid-positive people with dementia. Because tau PET correlates with disease stage, it might be useful for selecting patients for trials and as a potential outcome measure, Knopman suggested.
Many talks noted that the spread of tangles depends on disease stage and follows specific spatial patterns. Agneta Nordberg of the Karolinska Institute, Stockholm, presented data from a small cohort of 38 people who ran the gamut from cognitively normal to AD. They underwent scans with tau tracer THK5317, developed by Tohoku University, Sendai, Japan. About half the group came back for follow-up THK5317 and FDG PET scans one to two years later. At the group level, brain metabolism waned, but THK5317 retention did not change. On the individual level, however, participants displayed heterogeneous regional patterns of change in THK5317 retention. In people in the prodromal stage, tau tangles built up mostly in the medial temporal lobe, while in those with AD, the pathology spread throughout the brain, Nordberg noted (see image below). In addition, the more brain amyloid a person had at baseline, the faster tau accumulated, she reported (see Chiotis et al., 2016).

Tracking Tau Accumulation. Tau PET ligands allow researchers to see regions (red) where tangles build up in an individual as prodromal Alzheimer’s progresses. [Courtesy of Agneta Nordberg.]
Hanna Cho of Yonsei University, Seoul, South Korea, and colleagues wanted to test whether the spread of amyloid and tau followed the patterns described by Braak staging, as previous PET studies suggest. At AAIC, Cho described a cross-sectional study that compared amyloid PET with florbetaben (trade name Neuraceq™) and AV1451 tau scans in 195 people who ranged from cognitively normal to AD. The researchers assumed that the brain regions that most commonly contain plaques and tangles were the sites of earliest accumulation of those proteins, while regions where pathology occurs less often would be those that succumb later in the disease. Using this logic to evaluate the data from her cohort, she determined that amyloid spread rapidly through the cortex, later reaching the medial temporal lobe, while tau accumulation followed a stepwise pattern through different regions, starting in the entorhinal cortex and spreading to temporal and parietal cortices before reaching the neocortex. Atrophy followed the same stepwise pattern as tau (see Cho et al., 2016). The pattern of tau accumulation mirrored Braak staging and disease severity and may serve as a useful biomarker, Cho suggested.
Tau Tangles Track with Atrophy and Cognition
Speakers at AAIC consistently reported that tau pathology correlated with declining cognition. Mintun noted that higher AV1451 SUVRs associated with cognitive decline in his cohort. In the Swedish cohort, high tau signal correlated with worse performance on tests of episodic memory, as well as poorer global cognition, Nordberg said.
Shannon Risacher of Indiana University School of Medicine, Indianapolis, reported similar findings from the Alzheimer's Disease Neuroimaging Initiative (ADNI). This long-running initiative added tau imaging only recently, scanning participants two to five years after they entered the study. Risacher compared baseline and longitudinal measures of brain Aβ accumulation, hypometabolism, atrophy, and cognition to these later scans with AV1451. She found that baseline Aβ best predicted a high tau signal. The next-best predictors of tau pathology were the rates of change in three measures: memory scores, the CDR-SB, and hippocampal volume. This agrees with prior PET findings tying tangles to cognitive decline and degeneration.
In several studies, tangles in specific brain regions correlated with atrophy and hypometabolism in that same area. In a cohort from the Australian AIBL study, comprising 89 controls, 24 people with mild cognitive impairment, and seven with dementia, those who had high tau signal in regions such as the hippocampus, entorhinal cortex, prefrontal cortex, and anterior cingulate also had smaller volumes in those same regions. This was true even in non-demented participants, said Vincent Doré of CSIRO, Canberra, Australia. In one case where a participant had more tau signal in the right hemisphere, atrophy was also localized to the right side, he noted. The researchers saw similar results with both AV1451 and THK5351, a successor to THK5317. “The association between regional tau and regional atrophy is already evident at the early stages of tau deposition,” Doré concluded.
Can tau accumulation flag the earliest, subtle memory impairments? Rachel Buckley of Harvard Medical School, Boston, addressed this in her talk. She scanned 106 cognitively normal AIBL participants using AV1451, assigning them to high and low MTL tau groups, and asked all if they had concerns about their memory. People in the high tau group tended to answer yes, and the tendency was even stronger in those with positive amyloid scans. High MTL tau correlated with poor episodic memory and weak executive function, and people scored even worse on cognitive tests if they also reported memory concerns. The results suggested that tau pathology may underlie subjective memory complaints. This might prove useful for staging preclinical disease, Buckley said, though she acknowledged the study was small, and a simple yes/no question might not capture the complexity of early cognitive problems. In amyloid studies, subjective cognitive concerns have been found to correlate with amyloid burden (see Amariglio et al., 2012; Sep 2014 news).—Madolyn Bowman Rogers
References
News Citations
- Improving Tau PET: In Search of Sharper Signals
- Staging of Alzheimer’s, the Second: Neurodegeneration Does Not Equal Tauopathy
- Tau Tracer T807/AV1451 Tracks Neurodegenerative Progression
- Do Tau Tracers Track Cognitive Decline in Disease?
- Scan by Scan, Growing Tau PET Data Picks Up Early Memory Deficits
- On Multiple Marker Analysis, Tangles Track Best With Functional Decline
- Tau PET Aligns Spread of Pathology with Alzheimer’s Staging
- Do Temporal Lobe Tangles and Cortical Plaques Together Bring on Alzheimer’s?
- Brain Imaging Suggests Aβ Unleashes the Deadly Side of Tau
- Memory Concerns Presage Cognitive Decline and Aβ Pathology
Paper Citations
- Chiotis K, Saint-Aubert L, Savitcheva I, Jelic V, Andersen P, Jonasson M, Eriksson J, Lubberink M, Almkvist O, Wall A, Antoni G, Nordberg A. Imaging in-vivo tau pathology in Alzheimer's disease with THK5317 PET in a multimodal paradigm. Eur J Nucl Med Mol Imaging. 2016 Aug;43(9):1686-99. Epub 2016 Mar 21 PubMed.
- Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, Lee JH, Ryu YH, Lee MS, Lyoo CH. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016 Aug;80(2):247-58. Epub 2016 Jul 8 PubMed.
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012 Oct;50(12):2880-6. PubMed.
Further Reading
News
- Tau Tracers Track First Emergence of Tangles in Familial Alzheimer’s
- Tau PET Fits With CSF, Grows Over Time, Picks up Frontotemporal Cases
- What If It’s Not Garden-Variety AD? Telling Variants Apart by Where Tau Is
- Tau Takes Center Stage at 10th Human Amyloid Imaging Conference
- At HAI, Researchers Explore Diagnostic Potential of a Tau Tracer
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.