References and Thresholds—Amyloid Imaging Protocols Debated at AAIC
Quick Links
Compared to tau imaging, PET scanning for amyloid may seem like ancient technology, but there is ample room for improvement. At the Alzheimer's Association International Conference 2014, held July 12-17 in Copenhagen, Denmark, researchers proposed changing imaging protocols to sharpen their diagnostic and prognostic potential and improve the power to track amyloid changes over time. The change might require re-analysis of some existing data sets.
In a poster presentation, Kewei Chen from Banner Alzheimer's Institute, Phoenix, made the case for using cerebral white matter as a reference region for Florbetapir PET imaging of brain amyloid. Most labs now use the cerebellum or pons when calculating standard uptake value ratios (SUVRs) to quantify Aβ deposits. As co-author Eric Reiman explained, much of the cerebellum and pons lie below cerebral sites where amyloid accumulates, such as the entorhinal cortex, hippocampus, and medial frontal cortex (see image below). As PET scanners "slice" through the brain, the cerebellum and pons rarely fall in the same plane as Aβ-positive tissue; in fact they lie at the bottom of the field of view of the PET camera, where a slight change in head position could introduce variability from scan to scan. "We think that scans taken lower in the brain introduce a lot of noise," said Reiman.
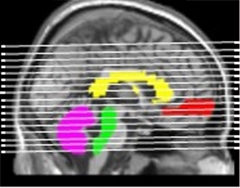
Location, location, location.
Cerebral white matter (yellow) makes a better reference for cerebral (gray) Aβ than the cerebellum (purple) or the pons (green), which lie below sites of mean cortical uptake of the amyloid tracker flobetapir (red). [Image courtesy of Kewei Chen, Banner Health.]
Chen and colleagues wondered if a reference region higher in the brain would improve the power to track amyloid change over time. They re-analyzed data from the Alzheimer's Disease Neuroimaging Initiative comparing the cerebellum, pons, and cerebral white matter as reference regions. Chen correlated the change in cortical SUVRs over two years to cognitive decline among 332 people (31 with probable AD, 187 with MCI, and 114 cognitively normal controls). Only SUVR changes calculated using white matter correlated with deterioration on the MMSE, ADAS-Cog, and CDR sum of boxes. Moreover, SUVRs calculated based on the reference regions varied less from person to person. For example, among people with AD who tested positive for amyloid at baseline, only the white matter reference method showed consistent increases in amyloid deposition in the cortex over the two years. Values based on the cerebellum and the pons fluctuated and suggested that some patients actually lost amyloid.
"The field is slowly coming around to the idea that for longitudinal measurements, white matter gives better signal and less noise," said William Jagust, University of California, Berkeley. In a pre-meeting imaging conference, Jagust reported highly variable changes in amyloid load among ADNI volunteers who started out with similar Aβ levels. That was partly due to problems calculating SUVRs. "The reference region was a real headache," Jagust said. His colleague Susan Landau, also from Berkeley, used the cerebellum as a reference, but that was suboptimal, said Jagust. Other regions, including the brainstem, the pons, and white matter, gave more consistent results.
Other groups are noticing similar trends. At AAIC, researchers from Avid Radiopharmaceuticals in Philadelphia reported that using white matter as a reference region increased the signal-to-noise ratio when measuring longitudinal changes in ADNI participants. In a poster, first author Abhinay Joshi showed that among amyloid-positive volunteers, baseline SUVRs correlated more tightly with two-year values when referenced against white matter (r=0.9) than cerebellum (r=0.73). The upshot was that for measuring change, white matter makes a better reference.
Does changing the reference region matter? Based on the two-year ADNI data, Chen and colleagues calculated how many patients might be needed to measure a 25 percent reduction in brain amyloid in a clinical trial. While about 8,000 Aβ-positive MCI patients would have to be enrolled to see an effect over one year using the cerebellar reference, 325 would suffice if the white matter was used instead. "The differences are staggering," said Reiman. "We believe that the use of a cerebral white matter reference region will greatly improve the possibility to track longitudinal changes in fibrillar amyloid PET measurements, relate them to clinical decline, and evaluate amyloid-modifying treatments in clinical trials." While additional studies are needed to clarify whether the findings apply to different amyloid PET ligands and PET scanners, Reiman suggested that researchers might want to re-analyze previous trial data with this approach.
Yet another measure that colors interpretation of imaging data is the threshold researchers use to classify a person as amyloid-positive or -negative. Many groups set an SUVR value of 1.4 or higher as being amyloid positive. In the preconference imaging symposium, Sylvia Villeneuve, who works with Jagust at Berkeley, claimed this number is too high. In an approach others praised for its elegance, she reanalyzed the rationale for this figure and concluded that it should be lower.
First, Villeneuve compared amyloid uptake among 154 cognitively normal old adults and a sample of young adults presumed to be amyloid-free. That analysis suggested that a Pittsburgh compound B (PiB) SUVR threshold of 1.19 predicts amyloid deposition. In another test, she looked at Gaussian distributions of SUVRs. That showed two major groups with low and high values that reflect amyloid-negative and -positive individuals. An SUVR of 1.22 equated to a 90 percent probability of being in the low or amyloid-negative range. She obtained the same value from a cluster analysis, where she looked at 74 different brain regions to see which discriminated individuals with and without amyloid and at what SUVR threshold amyloid began to appear.
Finally, Villeneuve reported a voxel-wise estimate. In this analysis, she took 22 people with the lowest SUVRs, compared them to 22 people with the next-highest SUVRs (the test group), and looked voxel by voxel for differences in amyloid. If none appeared, she added the person with the next-highest SUVR into the test group (removing the person with the lowest SUVR) and re-ran the analysis. She kept this "sliding-window approach" going until she detected a difference between the two groups. That occurred when the SUVR reached 1.19.
Villeneuve used this data to suggest that lower thresholds would better capture people who are Aβ positive. Cliff Jack, Mayo Clinic, Rochester, Minnesota, was concerned about false positives and said that lower thresholds might only work for specific amyloid ligands and specific algorithms. "If we used a threshold of 1.2, everyone over 50 would be amyloid-positive," he said.
Jagust, working with Gil Rabinovici at the University of California, San Francisco, correlated thresholds with pathological data from about 50 cases of dementia/MCI and a few controls. At AAIC, he reported that the lower threshold SUVR values for PiB are highly specific. "We were surprised it did not lead to more false positives," he said. On top of this, the sensitivity increased to 80 percent, from 60 percent for the high-threshold SUVR. "The literature suggests sensitivity is mid- to high 90s for the higher-threshold values," he said. When he applied the higher SUVRs reported by others, the sensitivity was lower. "If we use the high-threshold values, we may miss a fair proportion of cases that have low numbers of plaques," he said.
Jagust agreed with Jack that many different parameters go into setting SUVR thresholds. The important thing is to pick the number rationally, not just follow figures reported in the literature. "If you want to find out how amyloid changes as people age, for example, then you may want to use a lower threshold," he said.
Victor Villemagne, Austin Health in Melbourne, Australia, used Villeneuve's lower SUVR threshold to estimate how long it takes people to develop memory problems once they become amyloid positive. He reported that in the Australian Imaging Biomarkers & Lifestyle Flagship Study of Ageing, memory impairment correlated with SUVRs of 1.4 and above. Based on a pathology threshold of 1.2 and a memory threshold of 1.4, Villemagne calculated a window of five to seven years’ time to intervene before people with the first wisps of amyloid in the brain start to notice memory problems.—Tom Fagan
References
No Available References
Further Reading
Papers
- Chen K, Roontiva A, Thiyyagura P, Lee W, Liu X, Ayutyanont N, Protas H, Luo JL, Bauer R, Reschke C, Bandy D, Koeppe RA, Fleisher AS, Caselli RJ, Landau S, Jagust WJ, Weiner MW, Reiman EM, Alzheimer’s Disease Neuroimaging Initiative. Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med. 2015 Apr;56(4):560-6. Epub 2015 Mar 5 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.