Plasma Aβ—First Sign of AD, But Tough to Measure Prospectively?
Quick Links
Scientists believe that poor clearance of Aβ from the brain underlies sporadic Alzheimer’s disease. Now, evidence for subtle changes in the plasma levels of Aβ1-42 seems to back that up. At this year’s Clinical Trials in Alzheimer’s Disease meeting, held November 9-12 in Boston and online, scientists led by Suzanne Schindler, Washington University, St. Louis, reported that the plasma Aβ42/40 ratio not only falls in people at the very earliest stage of the disease, it does so years before that ratio drops in the CSF.
- Aβ42/40 falls in blood before it falls in CSF.
- Poor clearance of Aβ42 from the brain may be first sign of AD.
- Plasma Aβ42/40: a robust assay to use prospectively?
Schindler’s tantalizing hypothesis is that at the point of origin of AD, the plasma Aβ42/40 ratio begins to fall not because Aβ42 peptide has begun to form plaques in the brain—the generally accepted hypothesis—but because less of it seeps out of the brain as people age. Only later does the peptide form plaques and soak up peptide that would otherwise show up in the CSF. If true, this nuanced view would peg plasma Aβ42/40 ratio as the very first marker to change in AD. This could have implications for the basic understanding of Alzheimer’s pathophysiology and potentially the way it is treated.
It could also support using the plasma Aβ42/40 ratio for screening in trials that aim to nip the disease in the bud. Alas, for that, this measure may be finicky. Data presented by Christina Rabe, Genentech, South San Francisco, threw cold water on the screening idea. She reported that because the plasma Aβ42/40 change is so subtle, any small bias or variance in measurements or pre-analysitical handling would generate such high false positive and negative calls that trial candidates could end up being misclassified. Others pushed back, claiming that highly accurate tests and good quality control can make the measure robust enough for screening in trials, and even for general diagnostic use. The Alzheimer's Clinical Trials Consortium's AHEAD 3-45 trial of lecanemab has already begun to use C2N’s CLIA-approved mass spectrometry test for plasma Aβ to prescreen volunteers before running amyloid PET scans.
Plasma First
Schindler’s analysis was based on the large dataset from the Knight ADRC at WashU of people who have been volunteering for the center's multiple, decades-long observation studies. First, she determined how well C2N’s test, called Precivity, captured amyloid positivity as judged by either CSF Aβ42/40 analysis or PET scans.
Among 1,085 volunteers who'd had lumbar punctures and 710 who'd had PET scans, Precivity scores correctly predicted amyloid status by either measure with an area under the curve (AUC, a statistical measure) of 90 percent accuracy. The test performed equally well in people who were cognitively normal and in those who were impaired. Schindler had reported on a subset of 158 of these people previously (Schindler et al., 2019; Aug 2019 news).
How would the test predict future amyloid status? Here’s where things got interesting. Schindler tracked 273 people who had initially tested negative for amyloid based on their CSF Aβ42/40 ratio. Curiously, about 40 of them had Precivity scores above the cutoff, i.e., were predicted to have brain amyloid plaques at that time. Were these false positives? Probably not. Over the seven subsequent years, the CSF Aβ42/40 ratio for most of these volunteers slowly sunk downward, such that their CSF now tests positive, or close to positive, as well. “This suggests to us that these were not false positives at all,” Schindler told Alzforum (see image below).
Plasma Before CSF. Among people who had tested negative (top, blue and purple dots) or borderline (green dots) for brain amyloid based on a CSF Aβ42/40 cutoff (horizontal dashed line) at baseline, those who had tested positive by Precivity cutoff (vertical dashed line) at baseline (upper right quadrant, top), were likelier to test positive by CSF seven years later. [Movie courtesy Suzanne Schindler, WashU.]
The data imply that the plasma Aβ42/40 ratio starts falling years before it does in the CSF. “This was surprising, because it is generally believed that changes in CSF Aβ are the earliest signs of AD,” Schindler told Alzforum.
Henrik Zetterberg, University of Gothenburg, Sweden, considers this data “super interesting.” He has some questions, too. “If Aβ shifts from the CSF into plaques, then how would that be visible sooner in the blood than the CSF?” he wondered. In fact, Schindler said plasma and CSF diverge before plaques begin to form. She thinks the Aβ42/40 ratio in the plasma drops because clearance of Aβ42 from the brain begins to wane even while CSF Aβ remains high.
Her data support this idea. She measured Aβ42 and Aβ40 levels in the CSF and plasma of 124 people in the ADRC cohort that was collected halfway through the seven-year follow-up. All still had high CSF Aβ42/40, indicating they were negative for amyloid in the brain. Intriguingly, when she looked at the rate of change in these volunteers, the CSF ratio was stable, but the plasma ratio had already begun to decrease. Looking at each peptide individually, she found that both Aβ42 and Aβ40 had increased in the CSF with age, meaning the ratio stayed the same. In the plasma, however, Aβ40 increased more with age than did Aβ42, suggesting that Aβ42 was being cleared less efficiently from the brain. “Plasma Aβ may be taking us back to the real genesis of Alzheimer’s, which is reduced clearance of Aβ42 from the brain,” Schindler proposed.
Prior data from Randall Bateman’s lab at WashU supports this idea. He found that Aβ is directly transported across the blood-brain barrier in people, and that this clearance slows by two- to fourfold as people age into their 70’s (Roberts et al., 2014; Patterson et al., 2015).
Kaj Blennow, also from U Gothenburg, was cautious. “If there was a drop in clearance, then why would it not be a drop for both Aβ42 and Aβ40?” he asked. He said more studies are needed to confirm what is going on. Zetterberg said this could easily be done, noting “There has been prior data suggesting that Aβ ticks up slightly in the CSF before plaques begin to form, and that would be in keeping with Schindler’s hypothesis.”
Exactly what might crimp Aβ42 clearance before plaques are even there to mop it up remains to be seen, but Schindler thinks what she found is no exception. “The interesting thing is that this seems to be happening [in the ADRC cohort], even those with high CSF Aβ42/40,” she said (see image below). Schindler and Bateman now believe that AD doesn’t necessarily start when plaques begin to deposit, but when clearance of Aβ42 begins to wane, setting the stage for incorporation of the peptide into plaques.
Clearance may be a function of age and other factors, including APOE genotype. “We would predict that we might not see the plasma Aβ42/40 ratio dropping in younger people,” Schindler said. People in the ADRC cohort ranged from age 59 to 79.
Prospective Measures
If a person's plasma Aβ42/40 ratio opens a window into AD a few years before CSF Aβ42/40, then it would be the earliest marker of AD pathophysiology. Does that also make it the best screening marker for clinical trials or suitable for diagnosis? This is the idea Rabe challenged at CTAD.
Working with Tobias Bittner at Hoffmann-La Roche, Basel, Switzerland, Oskar Hansson at Lund University, Sweden, Zetterberg, Blennow and others, Rabe evaluated how well the plasma Aβ ratio works as a screening tool and how robust the measure might be in a prospective setting. All these authors are stakeholders in different plasma tests for Aβ and for various forms of phospho-tau.

Plummet in Plasma. Change in plasma Aβ42/40 over a period of up to 15 years. Each line represents repeat samples taken from one of 362 people in the ADRC cohort. The black line represents their baseline measure and each dot represents a different sample collection. Colors reflect the spectrum of baseline CSF Aβ42/40 values, with purple being the highest and red lowest. Arrowheads denote the last plasma sample and show that the ratio is falling in almost all participants, regardless of their initial CSF ratio. [Courtesy Suzanne Schindler, WashU.]
To date, many labs have tested the marker and found that it can distinguish people with AD from controls and from people with other diseases with remarkable accuracy, but most of those studies were done retrospectively, by analyzing a whole collection of samples together in one fell swoop, with the same batch of reagents (Aug 2018 news). Because the ratio changes by only 10-15 percent when a person develops amyloid pathology, clinical chemists doubt the plasma Aβ ratio is robust enough to use prospectively, on a day-to-day basis for diagnostic or screening purposes. In those settings, a person comes in, gets blood drawn, has it analyzed, and the doctor makes a call about whether he or she has brain amyloid or not. For that to work for a large trial, for example, the test needs to be perfectly stable over the course of a year or so while it enrolls.
Rabe modeled the idea of prescreening for a trial in data from the Swedish BioFinder study and from ADNI, using her company's Elecsys test. First, she asked if a plasma Aβ42/40 cut-off could be found that could work as a screening tool. The issue here is not only that the ratio changes very slightly in people who have brain amyloid, but also that there is considerable overlap between the “positives” and “negatives.” Rabe found that in BioFinder, where the AD prevalence is about 15 percent, a cutoff of 0.13 would rule out 45 percent of amyloid-negatives, while only eliminating 2 percent of those who were amyloid-positive (see image below). That would cut the number of PET scans needed to recruit amyloid-positive people into a trial by about 40 percent, Rabe said—a cost savings.
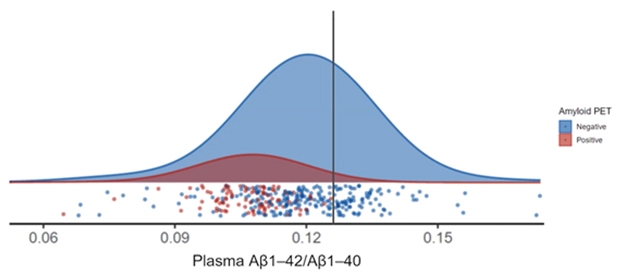
Ruled Out. In this model, use of a carefully selected plasma Aβ42/40 cutoff (vertical line) could avoid negative PET scans for a majority of amyloid-negative people, enriching trial populations for people who are amyloid-positive. [Courtesy of Christina Rabe, Genentech.]
But that calculation is based on retrospective analyses—a whole batch of BioFinder samples analyzed together. Would it work one-by-one going forward? Here, Rabe was concerned about how a plasma Aβ biomarker tolerates any variance in pre-analytical sample handling, or slight batch-to-batch changes in reagents that might creep in over time and skew the results.
The large overlap between amyloid-positives and -negatives and the narrow dynamic range in this assay means that a 10 percent bias could end up shifting a large proportion of the people above or below the selected cutoff, misclassifying them (see image below). A similar bias in CSF Aβ42/40 or plasma p-tau/Aβ42 would misclassify far fewer people.

Darned Drift. With plasma Aβ42/40 measurement, any slight variation due to assay bias or sample handling could misclassify people whose result lies near the cutoff (top line). For CSF Aβ42/40 and p-tau ratios, variability has less dramatic effects because the fold change between positivity and negativity is larger. (Amyloid PET positive: blue; negative: red). [Courtesy of Christina Rabe.]
Does this matter? Rabe thinks yes. “Assay conditions will never be perfect and a small bias or random variability should not result in a reclassification of patients,” she said. Bittner told Alzforum that the crux of the matter is how much error an assay can tolerate and still perform. “We see for Aβ42/40 that there is very little wiggle room for bias or variability, which would render the assay useless,” he said. “In fact, it can be so bad that you select the wrong people and deselect the right people.”
Bittner said he was taken aback when he first saw this. “We had a hard time believing the assay was not performing prospectively. We thought it was just a problem with our Elecsys system, but when we saw data for head-to-head comparisons on the ADNI site we realized that the dynamic range is no better for any of the other assays.”
Does this issue rule out plasma Aβ42/40 as a screening marker? The risk is great that it will not work, said Blennow. As he sees it, the problem is not the technique, but the marker itself. “The fold change is so small, the groups are very close together, and you put the cutoff right in the middle,” he said. Bittner agrees, saying “Our message is that it will not work prospectively, at least over the long term. It might be good for a few weeks or months, but once bias is introduced, performance will suffer.”
Bittner said the maximum bias a plasma Aβ42/40 assay could tolerate would be 3 percent. This is near the precision of mass spec assays used in Bateman’s lab at WashU and at C2N Diagnostics, which have the only CLIA-approved plasma Aβ42/40 test. Bateman was a co-founder of C2N. These assays outperform, in retrospective analyses, immunoassay-based methods and other mass spec assays, including the ones used by Roche and the U Gothenburg team (Oct 2021 news).
Joel Braunstein, C2N Diagnostics, insists plasma markers can be measured with great precision and that C2N's mass spec assay is robust. Precivity is the company's first commercial product. Braunstein told Alzforum that C2N has not modified its cut point since they rolled out the CLIA-approved test last year. “As part of our ongoing QC, we watch for it and evaluate the appropriateness of the cut point. To date, we have never seen evidence that we should modify it. Our experience is that we can absolutely distinguish individuals with and without disease and use it in a manner that would clearly provide value in prospective trials an even in clinical use,” he said.
The argument is more than academic. The AHEAD 3 and AHEAD 45 trials have begun to use this marker. Both trials are targeting cognitively normal people who are in the earliest stages of AD and are as young as 55 (Nov 2020 news). A3 enrolls people who fall between 20 and 40 centiloids on amyloid PET, while A45 targets those above 40 centiloids. At CTAD, Reisa Sperling, Brigham and Women’s Hospital, Boston, showed that AHEAD, which started screening in the middle of the COVID pandemic, soon noticed that the screen failure rate by amyloid PET was quite high, especially among those younger than 65. To bring that down, the investigators turned to C2N’s assay, hoping to enrich for people who are likely to be amyloid-positive.
As of October 18, AHEAD had screened 659 people for plasma Aβ42/40 in a retrospective batch. Everyone had also had an amyloid scan with the NAV4694 tracer. Sperling reported that those whose amyloid PET was above 20 centiloids had lower plasma Aβ42/40 ratios, suggesting that a cutoff strategy would work in this cohort. The plasma test also picked up most of the A3 participants, who fell into the 20-40 centiloid range (see image below).
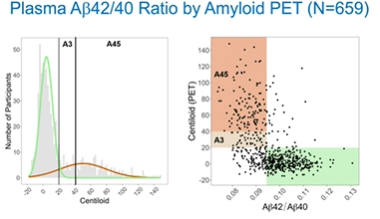
Fit for Prescreen? In AHEAD 3-45 trials, the Precivity test identified most people who tested positive for amyloid on PET. [Courtesy of Reisa Sperling.]
What about the test's robustness for prospective use? AHEAD investigator Paul Aisen, University of Southern California, San Diego, acknowledged the concern. He told Alzforum that AHEAD will re-evaluate the plasma Aβ42/40 cutoff point in these cohorts as the trial proceeds. “Because our process allows us to look at the relation of the cut-point to diversity of the population, the number of false negatives, as well as the screen fail rate on PET, there are a number of measures of the efficiency of the screening process that we will consider as the trial moves forward.” In her talk, Sperling said that AHEAD will initially err on the side of sensitivity to ensure that it misses neither potential A3 participants, who would be expected to have a smaller drop in the Aβ42/40 ratio, nor people from diverse backgrounds.
Zetterberg thinks this iterative process may take care of variability or bias that might creep into the test over time. “This approach could work, but it’s not the way clinical labs normally conduct assays, and it may be very demanding,” he said, adding that this would not be feasible if the test was to be rolled out as a routine diagnostic.
Jeffrey Dage, Indiana University School of Medicine, Indianapolis, believes a plasma Aβ assay can work. “This is an analytical and statistically heavy issue,” he wrote to Alzforum. “Robust and reliable plasma Aβ42/40 measurements need to be very high precision and the assay has to be kept in control.” Still, he believes plasma Aβ42/40 has potential for use in screening for clinical trials. “I am confident that it can work for trial enrichment and improve efficiency for preclinical AD studies, but it needs to be implemented carefully and managed closely,” he wrote. In his talk at CTAD he noted that the crux of the matter is effect size. “It’s really too small to use on its own for patient selection,” he said, but he thinks it could be used to enrich a population for a clinical trial.
Is this debate moot if those selected people are going to be scanned for brain amyloid anyway? Not if the people who are screened out after giving blood are not followed up. “We’ll never know what we missed in those who were excluded,” said Zetterberg.—Tom Fagan
References
News Citations
- Are Aβ Blood Tests Ready for Prime Time?
- With Sudden Progress, Blood Aβ Rivals PET at Detecting Amyloid
- In Side-by-Side Test of 8 Blood Aβ Assays, Mass Spec Shines
- BAN2401 Forges AHEAD into Phase 3, Preclinical AD
Paper Citations
- Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TL, Xiong C, Fagan AM, Bateman RJ. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019 Oct 22;93(17):e1647-e1659. Epub 2019 Aug 1 PubMed.
- Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, Moran CJ, Cross DT 3rd, Derdeyn CP, Bateman RJ. Amyloid-β efflux from the central nervous system into the plasma. Ann Neurol. 2014 Dec;76(6):837-44. Epub 2014 Oct 24 PubMed.
- Patterson BW, Elbert DL, Mawuenyega KG, Kasten T, Ovod V, Ma S, Xiong C, Chott R, Yarasheski K, Sigurdson W, Zhang L, Goate A, Benzinger T, Morris JC, Holtzman D, Bateman RJ. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015 Sep;78(3):439-53. Epub 2015 Jul 20 PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
C2N Diagnostics
C2N Diagnostics
Washington University
Washington University School of Medicine
C2N Diagnostics
Plasma AB-First Sign of AD, but Tough to Measure Prospectively?
At C2N Diagnostics (St. Louis, Missouri), we were very pleased to see at this year’s CTAD meeting the excellent progress being made in the field to incorporate blood-based biomarkers (BBBs) into prospective intervention studies for purposes of prescreening, obtaining pathological evidence of disease, and therapeutic monitoring. The goal for using BBBs in these studies is to speed enrollment, lower costs, reduce screen failure rates, dynamically track responses to therapy, and, ultimately, bring safe and effective treatments to patients more quickly.
The PrecivityAD™ plasma test is an analytically validated, CLIA-certified, laboratory-developed test currently being used in clinical care to aid Alzheimer’s diagnosis and clinical trials as a prescreener to improve enrollment efficiency. The test uses high-precision, liquid chromatography mass spectrometry (LC-MS/MS) to quantitate plasma Aβ1-42 (Aβ42) and Aβ1-40 (Aβ40) levels, expressed as a ratio, along with the ApoE proteotype (analogous to genotype) (Kirmess et al., 2021). Combined with age, these LC-MS/MS analyte measures are incorporated into a predictive algorithm that calculates an amyloid probability score (APS) on a scale of zero to 100. Multiple independent studies, including the work of Suzanne Schindler and colleagues (Schindler et al., 2021) and Reisa Sperling on behalf of the AHEAD 3-45 investigators (Sperling et al., 2021), presented at this year’s CTAD, have shown that the Precivity™ Aβ42/40 ratio test and the APS accurately detect the presence of brain amyloidosis and predict conversion to brain amyloid positivity many years prior to a positive amyloid PET scan or abnormal CSF Aβ42/40 ratio (Schindler et al., 2021; Sperling et al., 2021; Schindler et al., 2019; Ovod et al., 2017; West et al., 2021; Janelidze et al., 2021; Hu et al., 2021).
In their CTAD poster presentation (Rabe et al., 2021), Rabe et al. from Genentech and Roche Diagnostics challenged the idea that plasma Aβ42/40 could be useful as a prospective screening tool due the effects that analytical variance, and particularly assay drift over time, would have on the application of a prospectively chosen cut-point to data collected in the future, especially as part of a clinical trial that may enroll individuals over a period of several years.
To stress the point, Figure 4 of Rabe’s et al. poster presentation (figure titled “Darned Drift” in this Alzforum article) highlighted a hypothetical scenario whereby plasma Aβ42/40 ratios shifted by adding 10 percent bias in opposite directions for the individual analytes of Aβ42 and Aβ40, resulting in an overall 22 percent upward shift in the ratio. By doing this simulation, the authors showed that the bias in the Aβ42/40 ratio would reclassify almost all patients as amyloid-negative, thereby causing a complete loss of assay sensitivity.
We agree that a 22 percent shift over time would be cause for concern. However, our real-world experience with our PrecivityAD™ test shows that the simulation described is not valid for C2N’s Aβ42 and Aβ40 values measured using our precise and accurate analytical methods. Model assumptions from Rabe et al. were possibly derived from immunoassay platform-based plasma Aβ42/40 assays, but they should not be generalized to C2N’s LC-MS/MS assay. We have now used the PrecivityAD™ test under CAP/CLIA standards for approximately 18 months. During this time, we have analyzed and monitored multiple batches of quality control (QC) samples, each batch being in use for between three and18 months. From our combined QC data (more than 6,000 data points), we have observed less than a 0.6 percent drift in the Aβ42/40 ratio over the 18 months of monitoring. Similarly, the coefficient of variation (CV) of the Aβ42/40 ratio for the QC samples over this period of time is on the order of 4 to 5 percent, much lower than the simulated bias from the Rabe et al. poster.
The PrecivityAD™ test is now in use at a growing number of clinical practice sites throughout the United States to aid in clinical AD diagnosis for individuals with cognitive impairment. Academic investigators, private-public consortiums, and multinational pharmaceutical company collaborators are incorporating the test into their prospective study protocols for purposes of prescreening, aiding diagnosis, tracking treatment responses, and gaining new insights into disease biology and epidemiology. Thus far, user feedback on the test’s performance has been highly encouraging.
References:
Kirmess KM, Meyer MR, Holubasch MS, Knapik SS, Hu Y, Jackson EN, Harpstrite SE, Verghese PB, West T, Fogelman I, Braunstein JB, Yarasheski KE, Contois JH. The PrecivityAD™ test: Accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin Chim Acta. 2021 Aug;519:267-275. Epub 2021 May 17 PubMed.
Schindler SE, Yarasheski K, West T . Performance of the PrecivityAD™ blood test in detection of brain amyloidosis in cognitively normal and cognitively impaired individuals. Oral presentation at CTAD 2021, Nov 9-12.
Sperling R, Johnson K, Zhou J . Introduction of plasma biomarker screening for the AHEAD 3-45 Study. Late-Breaking Oral Communication at CTAD Meeting 2021, Nov 9-12.
Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TL, Xiong C, Fagan AM, Bateman RJ. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019 Oct 22;93(17):e1647-e1659. Epub 2019 Aug 1 PubMed.
Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, Sullivan M, Paumier K, Holtzman DM, Morris JC, Benzinger T, Fagan AM, Patterson BW, Bateman RJ. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017 Aug;13(8):841-849. Epub 2017 Jul 19 PubMed.
West T, Kirmess KM, Meyer MR, Holubasch MS, Knapik SS, Hu Y, Contois JH, Jackson EN, Harpstrite SE, Bateman RJ, Holtzman DM, Verghese PB, Fogelman I, Braunstein JB, Yarasheski KE. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021 May 1;16(1):30. PubMed.
Janelidze S, Teunissen CE, Zetterberg H, Allué JA, Sarasa L, Eichenlaub U, Bittner T, Ovod V, Verberk IM, Toba K, Nakamura A, Bateman RJ, Blennow K, Hansson O. Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021 Nov 1;78(11):1375-1382. PubMed.
Hu Y, Kirmess KM, Meyer MR, Rabinovici GD, Gatsonis C, Siegel BA. Association Between Amyloid Probability Score and Amyloid PET: A Clinical Validity Study. Manuscript Submitted 2021
Rabe C, Bittner T, Jethwa A. Utility of plasma Aβ1-42/Aβ1-40 as a screening tool is limited due to lack of robustness. Poster presentation at CTAD 2021, Nov 9-12.
Indiana University School Of Medicine
The timing of biomarker changes and their ultimate relationship with the pathobiology of Alzheimer’s disease will need additional study as the factors that influence each marker and measurement are likely to be different. The prognostic use for the plasma Aβ42/40 ratio in determining onset of pathology should be evaluated with additional prospectively designed longitudinal studies.
Christina Rabe’s analysis and message was very similar to mine. In determining clinical use of a biomarker assay, it is important to consider both the biology (effect size) and the analytical performance of the assay(s). Most of the ELISA methods evaluated for plasma Aβ40 and Aβ42 measurements have analytical performance with good precision at less than 10 percent, but because you have two independent methods your combined error is a problem when compared to the biological effect size.
An interesting thing to consider that was not addressed in Rabe’s talk or in the recent paper by Janelidze et al. is the method of measurement and reduction of total error that is achieved by using methods such as C2N’s (Janelidze et al., 2021). I don’t believe the IP/MS measurements of Aβ42 and Aβ40 are completely independent and thus the contribution to total error is less than when you make independent ELISA measurements. Perhaps there are further improvements possible in measuring the ratio of Aβ42/40?
There is significant risk to clinical research if care is not taken in implementation of plasma Aβ 42/40 and this will likely lead to doubt and skepticism, but the data support the potential use in preclinical AD to enrich a population by screening out subjects that have a high likelihood of being amyloid negative. The negative predictive value of the Aβ42/40 test can be quite good in this population and the cut point can be set to limit false negatives.
References:
Janelidze S, Teunissen CE, Zetterberg H, Allué JA, Sarasa L, Eichenlaub U, Bittner T, Ovod V, Verberk IM, Toba K, Nakamura A, Bateman RJ, Blennow K, Hansson O. Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021 Nov 1;78(11):1375-1382. PubMed.
Michigan State University
I find the data from the Rabe presentation comparing positive and negative histograms illuminating. It would be interesting to see these same histograms for the other studies being performed with different detection methods. Importantly, the greatest separation was found when including a p-tau measure in CSF. Would this improve the blood measures enough to increase the separation/effect size in these groups?
A final comment is that the drift for the ratio measure of Aβ is likely much less than for the absolute value of Aβ42 or Aβ40 as they should be modified by preanalytical variables similarly, and likely suffer similarly from some analytical variance as well.
Columbia University
Reduced Aβ42/Aβ40 ratio and an increase in either p-tau217 or p-tau181 in plasma were independently associated with developing a clinical diagnosis of AD in the multi-ethnic Washington Heights, Inwood Columbia Aging Project (Brickman et al., 2021) . The association between plasma biomarker concentrations derived at blood draw and the subsequent clinical AD diagnosis was approximately four years later. This indicates that these asymptomatic individuals already had the underlying biological changes associated with AD.
References:
Brickman AM, Manly JJ, Honig LS, Sanchez D, Reyes-Dumeyer D, Lantigua RA, Lao PJ, Stern Y, Vonsattel JP, Teich AF, Airey DC, Proctor NK, Dage JL, Mayeux R. Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021 Feb 13; PubMed.
Make a Comment
To make a comment you must login or register.