New Assay, New Cohorts—Plasma p-Tau181 Looks Even Better
Quick Links
The conference may be virtual, but the data is real. At the online AAT-ADPD 2020, researchers presented their talks and posters in much that same way as they would have in person—albeit without the open question-and-answer time. Up today was a presentation on plasma markers by Kaj Blennow, University of Gothenburg, Sweden. He showed that a new blood immunoassay his group developed for p-tau181 may be the most sensitive yet. It robustly detected a steady rise in plasma levels of the marker as Alzheimer’s disease progressed. Plasma p-tau181 distinguished people with AD from healthy controls and from those with frontotemporal dementia and other primary tauopathies with a high degree of accuracy, Blennow reported. Coming from 1,131 samples, the data corroborate findings recently reported in two Nature Medicine papers. Those studies used a proprietary p-tau181 assay developed by Jeffery Dage at Eli Lilly and Company, Indianapolis.
- A new immunoassay for p-tau181 may be the most sensitive yet.
- Works on Simoa platform using commercially available antibodies.
- It distinguished Alzheimer’s from healthy controls and other dementias.
“It is always comforting when we have slightly different assays that give the same result,” Henrik Zetterberg, UGot, told Alzforum. A co-author on all three papers, Zetterberg said both assays work beautifully. “I didn’t think measuring phospho-tau in the blood would be possible, but this has been super straightforward,” he said.
What has become known as the Dage assay runs on the Meso Scale Discovery platform. Owned by Lilly, it works like an ELISA, using capture and detection antibodies, but electrochemiluminescence rather than colorimetry quantifies binding of the latter for higher sensitivity. This assay is proprietary. Blennow and colleagues developed their own immunoassay on a single molecule array platform. Simoa is generally more sensitive than MSD. They used the same capture antibody as Dage, the AT270 mouse monoclonal, which recognizes phospho-threonine at position 181 of tau. For detection, the Gothenburg test uses the commercially available Tau12. It binds the N-terminal sequence QEFEVMEDHAGT, which begins at amino acid six of human tau. The Dage assay uses an anti-tau monoclonal developed by Lilly for detection. It also binds the N-terminal, but the epitope is just a little bit different, said Zetterberg. Researchers in Japan previously reported a p-tau181 immunoassay, but it reversed the antibody steps, using a total-tau antibody for capture and a p-tau181 antibody for detection. It did not detect p-tau181 in 11 of 15 control samples and four of 20 AD samples (Tatebe et al., 2017).
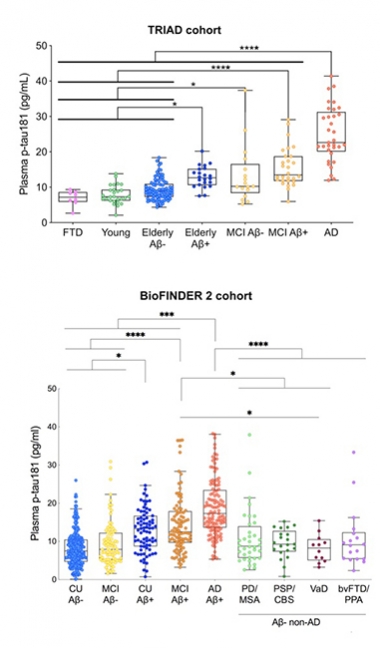
In Agreement. In the Triad and BioFINDER 2 cohorts, plasma p-tau181 tracks with severity of AD progression. [Courtesy of Kaj Blennow, University of Gothenburg.]
In his AAT-ADPD presentation, Blennow reported that the new assay’s so-called “lower limit of quantification” lies at 0.5 pg/mL. This means that p-tau181 was measurable in all 1,131 samples, even those from healthy controls as young as 23.
By contrast, in their Nature Medicine paper, researchers led by Adam Boxer at the University of California, San Francisco, reported an LLOQ of 1.4 pg/mL for the Dage assay. They were unable to detect p-tau181 in 41 of 404 samples from three cohorts (Thijssen et al., 2020). Similarly, Oskar Hansson’s group at Lund University, Sweden, found that of 589 samples, 20, including two from Aβ-positive individuals, fell below the level of detection using that same assay (Janelidze et al., 2020; Mar 2020 news). Level of detection is a less stringent metric than LLOQ.
Whether this small difference has practical consequences remains to be seen. “The Simoa assay may be more sensitive, but I don’t think it matters all that much, since we have so few samples below the limit of detection,” said Hansson. Zetterberg cautioned that it is hard to compare the analytical sensitivity of the two assays based on their LLOQs, since they are not calibrated or standardized to each other.
What does the new Simoa assay tell us? Thomas Karikari at UGot, working with Tharick Pascoal from McGill University, Montreal, measured p-tau181 in four different cohorts. For discovery, they tested 19 people with AD and 18 age-matched controls from UGot. For validation they used 226 volunteers from the Canadian TRIAD and 763 from the Swedish BioFINDER-2 prospective studies, respectively. The fourth set was 105 people from primary care; they included young healthy controls and people with MCI or AD. The study was a collaboration with Hansson’s group and with Pedro Rosa-Neto’s lab at McGill.
In the discovery cohort, the assay detected p-tau181 in both plasma and serum. “The assay works equally well in both,” Blennow said. Levels were elevated in AD compared with controls, and the same was true in TRIAD and BioFINDER-2 (see image above). In TRIAD, people who were Aβ-positive had higher plasma p-tau181 than did young controls, older Aβ-negative volunteers, and people with FTD. Old people, both cognitively normal or with MCI, had more plasma p-tau181 if they were Aβ-positive. People with AD had the highest plasma p-tau181 levels; their mean was 24.9 pg/mL. The marker distinguished AD from FTD, cognitively normal, and MCI with AUCs of 1.0, 0.95, and 0.85 respectively.
BioFINDER-2 told the same story. Plasma p-tau181 ticked up across the disease spectrum as diagnosis changed from Aβ-negative normal/MCI, to Aβ-positive normal/MCI, to AD. Plasma p-tau181 distinguished AD from PD/MSA, PSP/CBS, VaD, and bvFTD/PPA with AUCs of 0.82, 0.88, 0.92, and 0.83 respectively.
As was reported in the two Nature Medicine papers, Blennow, too, found that plasma p-tau181 as measured by the new assay was strongly correlated with CSF p-tau181 and with neurofibrillary tangle burden as judged by tau PET; AUCs for these correlations were high, at 0.91, 0.92, and 0.94 for SUVRs in Braak stages I-II, III-IV, and V-VI, respectively. In keeping with the idea that p-tau181 is an early response to Aβ toxicity, plasma levels among tau PET-negative volunteers were higher if the person was Aβ-positive. “This suggests plasma p-tau181 is an indicator of very early brain amyloidosis,” said Blennow.
In a separate talk, Karikari reported data from the 105 people in the primary care setting. P-tau181 was higher in the cognitively normal elderly than in young controls, and higher again in those with mild cognitive impairment. Values were highest in AD samples, where levels discriminated against the young, people over 60, and MCI with AUCs of 1.0, 0.84, and 0.55, respectively. The overlap between the AD and MCI patients suggests that the latter should be selected for specialist care, said Karikari.
To bolster this primary care data, Karikari analyzed 312 samples from the European AddNeuroMed prospective study. This public-private partnership coordinated by Simon Lovestone at King’s College London correlates blood marker data, cognition, and MRI analysis. Here, too, p-tau181 levels were lowest in samples from cognitively unimpaired, higher in MCI, and highest in AD samples. It correlated with clinically diagnosed AD with an AUC of 0.922, which was higher than the 0.830 recorded for neurofilament light, another promising marker for AD and other neurodegenerative diseases. The Boxer and Hansson groups also found that p-tau181 outperformed NfL in distinguishing AD from controls and from people with other neurodegenerative diseases. In fact, layering NfL on top of p-tau181 added no benefit, Hansson reported.
Blennow said this work is slated for publication in the April 22 Lancet Neurology—slightly delayed thanks to COVID-19.
All told, these three latest papers detailing separate analyses of more than 2,000 blood samples paint plasma p-tau181 as a noninvasive, robust, and specific marker of Aβ toxicity. “Plasma P-tau181 may be used as a first line of testing to identify patients likely to be tau-positive when tested by PET or CSF biomarkers, either to distinguish AD from other non-AD neurodegenerative diseases in cases with mild to moderate dementia or to predict future development of AD in cases with mild cognitive impairment,” wrote Hansson and colleagues.
Zetterberg thinks blood p-tau181 is ideal for monitoring responses to experimental drugs. “It almost seems that a successful therapy would have to attenuate p-tau181 levels,” he said. This could be tested in ongoing and even in completed trials. Companies have thousands of plasma samples from prior trials. “You would think they would assay for p-tau181 immediately,” Zetterberg said. He has asked for samples but has not yet received any.
Is a clinical-grade p-tau181 test far off? Zetterberg is convinced that this data is real and will hold up. He said the next step will be a fully automated, sensitive assay that could be rolled out in the clinic for diagnostic use.
Hansson agreed. “We need a reliable assay that works anywhere in the world, akin to the Elecsys or Fujirebio platforms used to measure CSF analytes,” he said. Roche, which sells the Elecsys system, is putting a lot of effort into this new marker, said Hansson. In parallel, the field will need to create a reference material that can be used to standardize tests, perhaps a tau fragment quantified by mass spectroscopy. “We need this type of development now,” said Zetterberg.
Could this push aside Aβ tests? Plasma Aβ42/40 falls by up to 15 percent in people with brain amyloid, a small change that is more difficult to measure accurately than p-tau181, which can more than quadruple. Different immunoassays for plasma Aβ42/40 don’t always agree either (Aug 2019 news). On top of this, immunotherapies that target free Aβ will bind it in blood, skewing plasma analysis. Despite these disadvantages, researchers still think plasma Aβ tests will be useful. “When we develop novel therapeutics, it makes sense to see how they affect amyloid,” said Zetterberg. He and Hansson both emphasized that Aβ changes will precede p-tau181 changes. “When we test very early, plasma Aβ42/40 may still offer an advantage,” said Hansson.—Tom Fagan
References
Antibody Citations
News Citations
Paper Citations
- Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, Kondo M, Allsop D, Tokuda T. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer's disease and down syndrome. Mol Neurodegener. 2017 Sep 4;12(1):63. PubMed.
- Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, Bourakova V, Cobigo Y, Heuer H, Spina S, VandeVrede L, Chai X, Proctor NK, Airey DC, Shcherbinin S, Duggan Evans C, Sims JR, Zetterberg H, Blennow K, Karydas AM, Teunissen CE, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Rabinovici GD, Dage JL, Rojas JC, Boxer AL, Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020 Mar;26(3):387-397. Epub 2020 Mar 2 PubMed.
- Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, Chai X, Proctor NK, Eichenlaub U, Zetterberg H, Blennow K, Reiman EM, Stomrud E, Dage JL, Hansson O. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020 Mar;26(3):379-386. Epub 2020 Mar 2 PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, Chamoun M, Savard M, Kang MS, Therriault J, Schöll M, Massarweh G, Soucy JP, Höglund K, Brinkmalm G, Mattsson N, Palmqvist S, Gauthier S, Stomrud E, Zetterberg H, Hansson O, Rosa-Neto P, Blennow K. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020 May;19(5):422-433. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.