Long COVID and Dementia: The Link Is Still Elusive
Quick Links
With the emergence of COVID-19 three years in the past, the lingering neurological effects after initial illness remain nebulous. At the recent International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden, scientists presented data from ongoing longitudinal trials studying the neurological effects of long COVID. Others tied the APOE4 genotype to higher odds of brain bleeds post-COVID. And there is data on how a protein from SARS-CoV-2, the virus that causes COVID, can gather within, and maybe inflame, the brain.
- A year after acute illness, some people report poor memory, executive function.
- At that point, nothing appears overtly wrong with their brain structure or metabolism.
- APOE4 carriers were prone to neurovascular complications of COVID.
- Spike protein found in brain tissue, skulls of people who died after having had COVID.
AD/PD featured a dedicated session on this topic, plus presentations throughout. The upshot? While certain neurologic symptoms are being reported subjectively, not all are measurable objectively. Affective issues present before infection appear to play a role, as well, making the problem difficult to define.
“The session emphasized the complexity of research into concrete neurological and pathophysiological sequelae of SARS-CoV-2 infection, as distinguishing de novo damage and predisposition is only possible in studies with well-characterized pre-morbid status,” wrote Michael Schöll, University of Gothenburg, who co-chaired the session.
Co-chair Michael Schlossmacher, Ottawa Hospital Research Institute, Canada, thinks that exploring COVID-related effects on the nervous system has implications for other neuropsychological disorders that result from interactions between viruses and genetic susceptibility. “The COVID-19 pandemic has unearthed the tip of an iceberg. What would Constantin von Economo say had he lived 100 years later?” Schlossmacher wrote. In 1917, this Greek-Austrian neurologist described encephalitis lethargica in the wake of that period's influenza pandemic (Kaya et al., 2016).
Best known for its respiratory symptoms, COVID can cause neurological changes, such as transient neurovascular damage and a spike in cerebrospinal fluid markers of neuronal injury, like neurofilament light (NfL) and glial fibrillary acidic protein (GFAP; Jan 2021 news).
At AD/PD, Jennifer Cooper in Cheryl Wellington’s lab at the University of British Columbia, Canada, reported seeing such a spike in NfL and GFAP in half the blood samples taken from 237 adults, ages 49 to 72, within a week after they were admitted to the ICU with COVID. High NfL and GFAP predicted who had neurological complications, such as ischemia or hemorrhage on CT scans, or who was likely to die, with areas under the curve of 0.77 and 0.82, respectively. AUCs measure sensitivity and specificity, with 1 being perfect. Cooper is following up these people at six and 18 months after hospitalization, collecting blood samples, MRI and PET scans, and testing their cognition.
Previously, some labs had reported that plasma NfL, GFAP, Aβ42, and total tau normalize six months after mild to severe COVID. Others still saw abnormal markers at six months in people who had had severe COVID, particularly with neurologic symptoms (Sep 2021 conference news). A recent systematic review of blood biomarker studies found elevated NfL and GFAP three to six-plus months post-infection in people with lingering neurological symptoms (see image below; Lai et al., 2023).
Also called “post-COVID condition” or “post-COVID syndrome,” long COVID broadly refers to continuation of symptoms, or appearance of new symptoms, three months after infection. Complaints include fatigue, shortness of breath, headaches, and difficulty with thinking or memory, aka “brain fog.”

Busy Biomarkers. People with long COVID have more NfL (red); inflammatory markers IL-6, TNF-α, CCL2; and SARS-CoV-2 antibody (Aβ) than people who fully recovered or never got sick (left). Biomarker changes depended on a person's symptoms, but high CRP was common to all (right). [Courtesy of Lai et al., 2023, Front Med (Lausanne).]
Is Long COVID Tied to Dementia?
The rise in neuronal injury markers begs the question of whether COVID increases one's odds of dementia later in life. Contracting herpes or influenza has been shown to increase the risk of developing dementia and other neurodegenerative diseases over 15 years (Feb 2023 news).
Fifteen-year data on COVID is a long way off, but some one-year data is in. Mild or severe bouts of COVID can, in some people, worsen existing neurological problems and speed cognitive decline in the year after infection (Apr 2021 conference news; Mar 2022 news). A study led by Ziyad Al-Aly at the Veterans Affairs St. Louis Health Care System last November reported much the same (Xu et al., 2022). Among 154,000 veterans who had COVID and 11.5 million who did not, the former were 1.4 times likelier than controls to have a neurologic condition—memory problems, a stroke, neuropathy, mental health disorders, migraines, or seizures—in the year following the illness. This was true for all COVID cases, from mild to severe.
Is there a link between “brain fog” during acute illness and lingering neurological problems? On May 5, Neil Wenger and colleagues at the University of California, Los Angeles, reported that among 766 adults, average age 60, who'd had COVID, 36 percent self-reported forgetfulness or trouble concentrating on the Perceived Deficits Questionnaire 30 days after hospitalization or outpatient clinic visit for COVID (Liu et al., 2023). Two months later, half of those people still reported neurological symptoms; one-quarter of people whose acute COVID had been without cognitive symptoms newly reported them. Prior depression, anxiety, or cognitive complaints were associated with these self-reports, leading the authors to propose an affective component to long COVID. This comports with an observation Schlossmacher noted, i.e., that a large fraction of patients with long COVID symptoms linked to nervous system dysfunction also had psychiatric symptoms, such as anxiety, depression, and adjustment difficulties.
To measure cognitive change in long COVID, Ann-Katrin Schild and colleagues at the University of Cologne, Germany, assessed global cognition and five domains in 42 adults, average age 45, with long COVID. All self-reported subjective cognitive impairment. Screening their cognition with the MMSE or MoCA picked up only one or 10, respectively, as impaired. This was true at three and nine months post-COVID. A composite of five cognitive domains—learning and memory, attention, executive function, visuoperception, and language—detected mostly mild impairment in 60 percent of participants at three months and 40 percent at nine. Both times, people had the most trouble with memory and executive function. When asked at AD/PD about the discrepancy between the greater subjective than objective cognitive assessment, Schild had no explanation.
The most comprehensive ongoing study of brain structure and function shown at AD/PD is that of Schöll and colleagues. They are measuring a broad range of dementia-related outcomes in 40 adults hospitalized with COVID and 20 controls who had never had it, all in their late 40s to mid-50s. Of the COVID patients, half were enrolled one month after admission to the hospital, half were enrolled between three and 20 months after hospitalization when they had been discharged but returned to an outpatient clinic for lingering neurological symptoms.
Participants underwent blood draws, lumbar punctures, structural and functional MRI scans, FDG PET scans, and extensive neuropsychological testing. The latter included the MoCA to measure global cognition, the Repeatable Battery for the Assessment of Neuropsychological Status for memory, and Trail Making tests to measure speed/attention and executive function.
At AD/PD, Schöll presented 12-month data on all but the fluid markers. At baseline, MoCA scores were the same between the three groups. After 12 months, the long COVID participants did marginally worse than controls, scoring an average of 25 versus 27, respectively. This indicated slightly impaired global cognition in those with PCC, as a score of 26 and above is normal.
In individual domains, people with long COVID had worse memory, executive function, and speed/attention than controls at baseline. People who had recently gotten COVID scored slightly worse only on the latter two. Speed/attention scores improved somewhat by 12 months; the other deficits did not change significantly (see image below).

Slooowly Getting Better? Over a year, speed/attention (right) improved significantly, memory (left) and executive function (middle) trended upward in people who had gotten COVID a month before enrolling in the study (top) or who had long COVID (bottom) before enrollment. The latter started with worse scores than the recently ill. [Courtesy of Michael Schöll, University of Gothenburg.]
MRI scans showed no differences in gray-matter volume in any group. People who more recently had COVID had larger white-matter hyperintensities than the earlier-discharged cases or controls at baseline; the number remained steady over 12 months. Intriguingly, radiologists noted that these hyperintensities were mostly widened perivascular spaces, which these people may have had before they contracted COVID. Schöll speculated that people with such hyperintensities might be predisposed to neurological symptoms.
On FDG PET, those with earlier COVID, but not the more recent cases or controls, at baseline had spots of hypometabolism in their precuneus, and clusters of hypermetabolism in their parietal cortices. In contrast, spots of glucose hyper- and hypometabolism popped up in the acutely ill, but not in the long COVID participants or controls, on their 12-month scans (see images below). Schöll noted that other researchers see similar patterns, but no one yet knows what to make of them. "Is it real? Is this compensating for an inflammatory response?" he wondered.
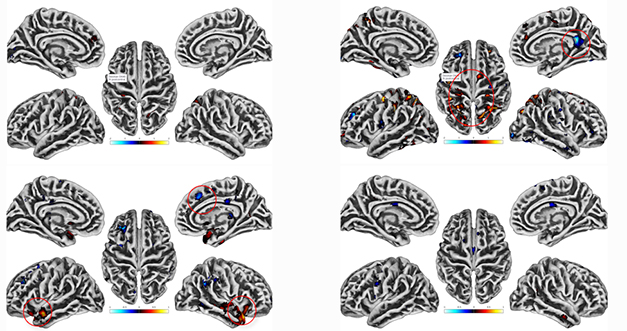
Perplexing PET. Compared to controls at baseline (top), people with long COVID (right) had spots of hypo- and hyperactive glucose metabolism (red circles), while people who'd had COVID recently did not (left). The opposite was seen at a 12-month follow-up (bottom). [Courtesy of Michael Schöll, University of Gothenburg.]
Overall, both Schild and Schöll found slightly worse memory and executive function in some people with long COVID, though the latter thus far is unable to find structural or functional explanations for the differences.
APOE and COVID
Pondering dementia risk invariably evokes the APOE4 allele, being, as it is, the largest genetic hit for sporadic AD. Some studies have shown that APOE4 carriers are likelier to show symptoms, become gravely ill, and die from COVID than APOE3 carriers, but others saw no influence of APOE genotype on COVID severity or outcomes (Jan 2021 news).
At AD/PD, data from Sophie Stukas, also in Wellington's lab at U British Columbia, fell into the latter group. Stukas genotyped blood samples from the same cohort of 237 people hospitalized with COVID that Cooper studies. Of those, 57 carried at least one APOE4; 180 did not. According to Stukas, an allele frequency of one-quarter is on par with E4 in the general Canadian population, meaning that APOE4 carriers were no more likely than noncarriers to get severe COVID.
Stukas found no differences in COVID symptoms, treatments, complications, length of hospital stay, or mortality based on APOE genotype. That said, E4 carriers were twice as likely to show neurological complications, such as ischemia or hemorrhage, on CT scans. Stukas did not say whether they had stroke symptoms. "This population may be more vulnerable to cerebral injury," she concluded.
This could be due to inflammation in the brain, at least in mice, said Ling Li, University of Minnesota, at AD/PD. Compared to human APOE3 knock-in mice infected with SARS-CoV-2, E4 knock-ins overexpressed RNA encoding the cytokines IL-6 and CCL2 and other proteins associated with the innate immune response to viruses. Li detected no SARS-CoV-2 viral RNA in the infected mouse brains, indicating that this inflammation occurred without direct viral brain infection.
Similarly, the SARS-CoV-2 spike protein, sans virus, was enough to trigger inflammation in cultured human brain cells treated with the viral glycoprotein. At AD/PD, Huyen Ngo in Hansang Cho’s lab at Sungkyunkwan University, Suwon, South Korea, reported that the spike protein binds to toll-like receptors 2 and 4 on the outer membranes of cultured human microglia and astrocytes. Binding evoked glial activation and anti-viral interferon signaling within the cells.
Li and Ngo's data mirror findings in people, namely the absence of detectable SARS-CoV-2 virus in postmortem COVID brain tissue despite a neuroinflammatory viral response (Jun 2021 news).
Tales from COVID Tissue In infected mice, the spike protein—again going solo—was spotted crossing the blood-brain barrier (Rhea et al., 2020). Could this be true in people? If so, could this explain some of the neuroinflammatory response to COVID?
Yes, according to researchers led by Ali Ertürk, Helmholtz Center Munich. Ertürk's group recently reported accumulations of the SARS-CoV-2 spike protein in cortical tissue, meninges, and skull bone marrow from 27 adults who had died from COVID (Rong et al., 2023). Mass spectrometry-based proteomics of their skull marrow showed downregulation of complement proteins and upregulation of pro-inflammatory cytokines compared to 10 control tissue samples. SARS-CoV-2 RNA was detected in only half the meningeal and skull samples that had spike protein. This led the scientists to suspect specific uptake, or a longer half-life, of this viral protein than the virus itself in the human brain.
Surprisingly, the scientists also found the spike protein in skull samples from 10 of 34 people who had died from something other than COVID in 2021 or 2022. This means they likely had had COVID in the past, and the spike protein lingered in the brain for the remaining months of their lives. Ertürk thinks that this loiterer might contribute to the neurological symptoms of long COVID.
Along similar lines, Ina Vorberg of the German Center for Neurodegenerative Diseases, Bonn, reported that the SARS-CoV-2 spike protein, as well as proteins from endogenous retroviruses, can help shuttle tau seeds between brain cells, possibly implicating the viral protein in tauopathies (Oct 2021 news; Apr 2023 news).
That said, spike must form trimers on cell membranes to enable tau spreading. “I do not find it likely that spike taken up by brain cells from the circulation would assemble into functional trimers,” Vorberg wrote to Alzforum. “However, a reservoir of spike protein in meninges and skull marrow could affect inflammatory responses in the CNS, which could affect protein aggregation and spreading by other means.”
Did people who perished after COVID have more neuropathology? At AD/PD, Schlossmacher shared early data from ongoing work. In piriform cortex and olfactory bulb tissue from 47 people who had died from COVID complications two to 12 weeks after infection, his group spotted tau tangles in 23 people and α-synuclein aggregates in 12; among 19 non-COVID autopsy controls, 11 had tangles, and none had α-synuclein aggregates. Schlossmacher said it's too soon to know if this was due to infection or age. The prevalence of olfactory bulb tau and α-synuclein aggregates in these Ottawa COVID cases was similar to that of a larger series of normal older adults from the Banner Sun Health Body Donation Program (Tremblay et al., 2022).
All told, the connection between COVID, neuropathology, and dementia risk remains murky despite efforts around the world to characterize the question. Longitudinal studies on neurological sequelae are continuing.—Chelsea Weidman Burke
References
News Citations
- How Does COVID-19 Affect the Brain?
- Aβ, Tau, and Other AD Markers Altered in COVID
- Nothing to Sneeze At: Viruses Raise Risk of Neurodegenerative Disease
- COVID-19 Worsens Neurological Problems, Delirium
- Mild COVID Infection Can Shrink Brain, Speed Cognitive Decline
- APOE Tied to Increased Susceptibility to SARS-CoV-2
- COVID-19 Prompts Choroid Plexus to Ring Alarm Bell
- Viral Proteins Help Shuttle Tau Aggregates Among Cells
- Attack From Within: How Ancient Viruses Resurface to Spread Tau
Paper Citations
- Kaya Y, Uysal H, Akkoyunlu G, Sarikcioglu L. Constantin von Economo (1876-1931) and his legacy to neuroscience. Childs Nerv Syst. 2016 Feb;32(2):217-20. Epub 2015 Feb 24 PubMed.
- Lai YJ, Liu SH, Manachevakul S, Lee TA, Kuo CT, Bello D. Biomarkers in long COVID-19: A systematic review. Front Med (Lausanne). 2023;10:1085988. Epub 2023 Jan 20 PubMed.
- Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022 Nov;28(11):2406-2415. Epub 2022 Sep 22 PubMed.
- Liu TC, Yoo SM, Sim MS, Motwani Y, Viswanathan N, Wenger NS. Perceived Cognitive Deficits in Patients With Symptomatic SARS-CoV-2 and Their Association With Post-COVID-19 Condition. JAMA Netw Open. 2023 May 1;6(5):e2311974. PubMed.
- Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2020 Dec 16; PubMed.
- Rong Z, Mai H, Kapoor S, Puelles V, Czogalla J, Schaedler J, Vering J, Delbridge C, Steinke H, Frenzel H, Schmidt K, Caliskan OS, Wettengel JM, Cherif F, Ali M, Kolabas ZI, Ulukaya S, Horvath I, Zhao S, Krahmer N, Tahirovic S, Yildirim AO, Huber T, Ondruschka B, Bechmann I, Ebert G, Protzer U, Bhatia HS, Hellal F, Erturk A. SARS-CoV-2 Spike Protein Accumulation in the Skull-Meninges-Brain Axis: Potential Implications for Long-Term Neurological Complications in post-COVID-19. 2023 Apr 05 10.1101/2023.04.04.535604 (version 1) bioRxiv.
- Tremblay C, Serrano GE, Intorcia AJ, Sue LI, Wilson JR, Adler CH, Shill HA, Driver-Dunckley E, Mehta SH, Beach TG. Effect of olfactory bulb pathology on olfactory function in normal aging. Brain Pathol. 2022 Sep;32(5):e13075. Epub 2022 Apr 29 PubMed.
Further Reading
Papers
- Edén A, Simrén J, Price RW, Zetterberg H, Gisslén M. Neurochemical biomarkers to study CNS effects of COVID-19: A narrative review and synthesis. J Neurochem. 2021 Oct;159(1):61-77. Epub 2021 Aug 20 PubMed.
- Wang L, Western D, Timsina J, Repaci C, Song WM, Norton J, Kohlfeld P, Budde J, Climer S, Butt OH, Jacobson D, Garvin M, Templeton AR, Campagna S, O'Halloran J, Presti R, Goss CW, Mudd PA, Ances BM, Zhang B, Sung YJ, Cruchaga C. Plasma proteomics of SARS-CoV-2 infection and severity reveals impact on Alzheimer's and coronary disease pathways. iScience. 2023 Apr 21;26(4):106408. Epub 2023 Mar 14 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.