How Does COVID-19 Affect the Brain?
Quick Links
Part 1 of a two-part series. Click here for Part 2.
Neurological symptoms, ranging from loss of taste or smell to life-threatening stroke, threaten many people with COVID-19. With some reports of neurological symptoms lingering for months after “recovery,” scientists are wondering what the long-term consequences of infection may be. Besides temporary lethargy, memory problems, and fuzzy logic, could COVID-19 increase risk of Alzheimer’s disease? Researchers have formed consortia to track neuropsychiatric outcomes.
- COVID-19 can cause neurological disease, uptick in neurodegenerative markers.
- In vivo and in vitro MRI detects transient brain damage.
- The virus can infect cultured brain cells and brain organoids.
- Consortium to track long-term neurological outcomes.
Scientists have only begun to investigate what is going on in the brain of a COVID-19 patient. Some report evidence that the SARS-CoV-2 virus infects brain cells in vivo, while others say it does not. fMRI of a small number of cases shows network dysfunction months after infection. Postmortem brains show signs of vascular damage, including microbleeds, blood vessel swelling, and hypoxic injury. Blood and cerebrospinal fluid show increased markers of brain injury, including the neuronal injury marker NfL. In vitro, the virus infects and distorts neurons, astrocytes, and choroid plexus epithelial cells, and causes phosphorylation of tau. Concerningly, perhaps, people who carry an ApoE4 allele may be at higher risk for SARS-CoV-2 infection and brain damage, adding to their already heightened risk for Alzheimer’s disease and other brain disorders (see Part 2 of this series). What do these hints amount to, and might they prime the brain for future neurodegeneration or even Alzheimer’s?
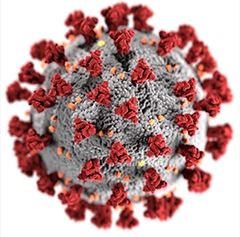
SARS-CoV-2. This illustration depicts the spike proteins (red) that create the distinctive corona on the outside of the virus. [Photo courtesy Alissa Eckert, Dan Higgins, CDC.]
SARS-CoV-2 in Cultured Brain Cells
Some clues to what might occur in the brain come from cultured cells and brain organoids. Researchers led by Akiko Iwasaki and Kaya Bilguvar at the Yale School of Medicine, New Haven, Connecticut, wanted to know if SARS-CoV-2 can infect these cells, and found that when they added it to human brain organoids, viral RNA turned up in neuronal progenitor cells (NPCs), neurons, and radial glia (Song et al., 2021). Likewise, Yanhong Shi, Beckman Research Institute, Duarte, California, and Vaithilingaraja Arumugaswami, University of California, Los Angeles, and colleagues reported that SARS-CoV-2 infects cultured NPCs, neurons, and astrocytes (Wang et al., 2021). Kwok-Yung Yuen, Jian-Dong Huang, and colleagues at the University of Hong Kong also saw viral infection of NPCs and neurons in cultured brain cells and brain organoids (Zhang et al., 2020). These studies hint that the new virus might directly infect cells of the CNS.
Others are not so sure. Scientists led by Guo-li Ming, University of Pennsylvania, Philadelphia, found that SARS-CoV-2 has almost no penchant for infecting human neurons or astrocytes in cell culture. However, the virus did take up residence in cultured epithelial cells of the choroid plexus, the brain’s major boundary between blood and cerebrospinal fluid (Jacob et al., 2020). This implies that the virus might access the entire CNS through these cells. Echoing this, researchers led by Madeline Lancaster, Medical Research Council Laboratory of Molecular Biology, Cambridge, England, U.K., found no infection in neuronal cells of brain organoids but robust infection in choroid plexus epithelial cells. The latter express ACE2, a cell-surface protein that SARS-CoV-2 uses as a portal to the cytosol (Pellegrini et al., 2020).
If SARS-CoV-2 does infect brain cells, what kind of damage might ensue?
When Jay Gopalakrishnan and Heiner Schaal at Heinrich‐Heine‐Universität, Düsseldorf, Germany, infected brain organoids with the virus, neurons ramped up phosphorylation of tau, which shifted from axons to soma, as has been documented for neurons harboring tau mutations that cause dementia. Some of the infected neurons died (Ramani et al., 2020). Iwasaki and Bilguvar also saw neurons die in organoids, where neighboring uninfected cells withered as well. In Shi and Arumugaswami’s experiments, the coronavirus killed neurons, and caused astrocytes to swell and their nuclei to crumble. The virus more readily infected APOE4 neurons and astrocytes than APOE3 cells (see Part 2 of this series). Lancaster and colleagues also saw tight junctions in infected choroid plexus epithelia break down. The brain organoids shrunk, leaking CSF-like fluid from within.
Do these responses in cultured tissue imply that SARS-CoV-2 can prime the brain for neurodegeneration or dementia? Infection by another virus, herpes simplex virus type 1, has been reported to trigger AD-like pathology, including hyperphosphorylated tau, amyloidosis, and neuron death (May 2020 news). “It will be important to explore how SARS-CoV-2 infection influences Aβ and tau accumulation in infected patients,” Takahisa Kanekiyo at the Mayo Clinic in Rochester, Minnesota, told Alzforum.
COVID-19 in the Human Brain
Do such in vitro data reflect what goes on in the brain? Scientists remain cautious. “There is no clear evidence of neuron or astrocyte infection in patients with COVID-19,” wrote Avindra Nath, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland, to Alzforum.
Still, some COVID-19 patients develop neurological symptoms, and case reports of neurovascular damage in those with severe COVID-19 are emerging. Sophie Demeret and colleagues at the Brain Institute Paris used MRI to peer inside the brain of a 69-year-old man hospitalized with COVID-19 who had suffered a seizure (Le Guennec et al., 2020). Hyperintensities in his right orbitofrontal cortex (OFC) indicated blood vessel damage and potential neuronal injury. The hyperintensities persisted for two weeks and resolved after 30 days. The authors could not determine if the damage was due to direct viral injury, post-infection encephalitis, or blood-brain barrier dysfunction.
Patients with severe COVID-19 are not the only ones with brain problems. Marco Grimaldi, Humanitas University, Milan, Italy, found subtle hyperintensities in the olfactory bulb of a 25-year-old woman with mild COVID-19 who had lost her sense of smell (Politi et al., 2020). Her olfactory bulb appeared normal again 28 days later. Khaled Gad, Suez Canal University, Ismailia, Egypt, reported much the same (Ismail et al., 2021). When a 25-year-old woman’s sense of smell and taste had not returned three months after having a mild case of COVID-19, doctors scanned her brain. Although everything in her MRI looked normal at rest, her OFC did not light up during a smell test (see image below). Olfactory signals are processed in the OFC.

Pass the Smell Test? Three months after having COVID-19, a 25-year-old woman’s brain looks normal on MRI (left), but her orbitofrontal cortex did not light up during a smell test (right), indicating faulty olfaction. [Courtesy of Ismail et al., JAMA Neurology, 2021. ©2021 American Medical Association. All rights reserved.]
Beyond case reports, postmortem series bore bleaker news. Xavier De Tiège’s team at Université libre de Bruxelles, Belgium, took MRI scans of 19 people within 24 hours after they had died with COVID-19, and found signs of microbleeds, including white-matter changes, in four of them (Coolen et al., 2020). Iwasaki, Bilguvar, and colleagues found microbleeds in brain tissue from three fatal COVID-19 cases. Likewise, Nath’s group spotted microvascular damage in postmortem brains from 13 people who had died from COVID-19 (Lee et al., 2020). Magnetic resonance microscopy and immunostaining of tissue slices identified swollen blood vessels and fibrinogen leakage indicative of microhemorrhages in the olfactory bulb.
Researchers led by Frank Heppner, German Center for Neurodegenerative Diseases (DZNE), Berlin, found tiny blood clots and damage to surrounding brain tissue in six of 33 people with COVID-19 (Meinhardt et al., 2020). For Isaac Solomon and colleagues at Brigham and Women’s Hospital, Boston, it was evidence of acute hypoxic injury in postmortem cerebral and cerebellar tissue of 18 people with COVID-19 that suggests the virus causes brain damage (Solomon et al., 2020). Under the microscope, they saw voids in the cerebral cortex, hippocampus, and cerebellum in all patients.
“The most compelling data linking COVID-19 to neuronal injury points to the brain vasculature,” Henrik Zetterberg, University of Gothenburg, Sweden, told Alzforum. “But the mechanism is not yet established.”
Does SARS-CoV-2 Worm Its Way into the Brain?
Prior evidence suggests coronaviruses can enter the brain in certain cases. Researchers previously isolated the related SARS-CoV-1 from postmortem brain tissue of a person who died during the first SARS epidemic in 2002-04, after experiencing severe central nervous system symptoms (Xu et al., 2005). To date, results for SARS-CoV-2 have been murky. Nath’s group found no viral RNA in postmortem COVID-19 brain tissue samples, but Solomon’s did detect nucleocapsid protein RNA in six of 32 brain samples from 16 donors. They found no virus in six other sample and low loads, defined as fewer than five copies of RNA per cubic millimeter of tissue, in the remaining 20. Nine of 20 samples taken from two other donors also have very low load. Likewise, Heppner and colleagues detected viral RNA in olfactory mucosa of postmortem tissue from 20 of 30 people with COVID-19. Others have looked for viral proteins. Iwasaki, Bilguvar, and colleagues found SARS-CoV-2 spike protein in cortical neurons of postmortem brain tissue from three people with severe COVID-19, suggesting the virus was replicating in brain cells. Intriguingly, spike protein lit up in spots where microbleeds were found.
To better understand the vulnerability of the brain, scientists have turned to animal models. During the first SARS pandemic, they made transgenic mice that overexpressed human ACE2. Unlike wild-type mice that developed only mild symptoms when infected, the transgenics developed severe disease and the virus flooded their brains (McCray, et al., 2007). Iwasaki, Bilguvar, and colleagues intranasally infected these hACE2 mice with SARS-CoV-2. The virus readily multiplied in the brain, appearing in neurons of the forebrain and cortex, though not cerebellum. Vascular endothelium cells escaped infection. Researchers led by Mukesh Kumar, Georgia State University, Atlanta, used the same mice and saw similar patterns. Six days after intranasal infection, the mice had festering brain infections. Hematoxylin and eosin staining of brain sections revealed shrunken, degenerating neurons. Peak brain virus loads correlated with onset of severe disease. The virus infected olfactory bulb cells, which could act as an entryway into the brain, they reported (Kumari et al., 2021).
A major advantage mice have over cell culture and brain organoids is their working blood-brain barrier. “The role of the BBB must be considered,” wrote Jessica Young, University of Washington, Seattle, adding “It is still unclear whether the neurological phenotypes in patients are due to direct infection or due to neuroinflammation because the BBB breaks down.”
William Banks, also at UWash, studied the BBB in infected mice. This team found that the virus’ spike protein, S1, crosses the BBB (Rhea et al., 2020). What does this mean for the brain? The authors postulate that proteins the viruses have shed in the periphery may trigger brain problems on their own. “The S1 protein in SARS-CoV-2 and the gp 120 protein in HIV-1 might function similarly: Both are glycoproteins, grab onto other glycoproteins on the cell surface, and cross the BBB,” wrote co-author Jacob Raber to Alzforum (comment below). “HIV’s gp 120 is toxic to brain tissue, so S1 is likely to be as well.” However, this remains to be seen in people.
Links to Biomarkers
In vitro and postmortem studies jibe with recent reports of CNS injury markers in people with COVID-19. Researchers led by Johan Virhammar, Uppsala University, Sweden, along with Zetterberg, detected more of the astrocyte activation/injury marker GFAP and the neuronal injury marker NfL in the CSF of 19 patients whose COVID-19 ranged from mild to critical (Virhammar et al., 2020; review by DeKosky et al., 2021). NfL and GFAP were up in 63 and 37 percent of patients, respectively. Sixteen percent also had elevated CSF total tau. NfL correlated with disease severity, neurological symptoms, and level of consciousness.
Another group led by Magnus Gisslén, University of Gothenburg, Sweden, also in collaboration with Zetterberg, focused on six hospitalized COVID-19 patients with neurological symptoms ranging from distorted taste to encephalopathy (Edén et al., 2021). Two of them had more NfL than normal in their CSF, while all six had higher levels of neopterin and β2-microglobulin—markers of cellular immune system activation. Curiously, other markers of CNS infection, such as white blood cell count, immunoglobulin G index, and albumin ratio, were normal.
Carlos Pardo and colleagues at Johns Hopkins University, Baltimore, saw no evidence of a cytokine storm or neuroinflammation, but NfL was elevated in the CSF of COVID-19 patients with stroke, encephalopathies, or headaches (Garcia et al., 2021). They compared CSF inflammation markers among 18 hospitalized COVID-19 patients, 14 healthy controls, and 68 non-COVID-19 neurological disease patients, including eight who had a stroke. A handful of pro-inflammatory cytokines and NfL had ticked up in both COVID-19 and stroke patients compared to controls. C-reactive protein was the only difference, being detected in seven of the 18 COVID-19 patients and only one of the 82 non-COVID-19 patients.
Another study led by Gisslén found higher GFAP and NfL in the blood of 18 ICU COVID-19 patients than in 33 controls (Kanberg et al., 2020). Raoul Sutter, University Hospital Basel, Switzerland, and colleagues also found more NfL in the blood of 29 critically ill COVID-19 patients compared to 10 critically ill non-COVID patients and 259 healthy controls (Sutter et al., 2020).
“Increased serum NfL is strongly associated with COVID-19,” said Zetterberg. “Now, we are teasing out to what extent CNS and peripheral nervous system injury contributes to this increase, because NfL is expressed in both.”
The Long Haul
The long-term consequences of COVID-19 are still anybody’s guess. Work from Andrea Pilotto, Alessandro Padovani, and colleagues at the University of Brescia, Italy, seems ominous. Six months after hospitalization, 55 of 165 severe COVID-19 patients reported lingering neurological symptoms, including fatigue, cognitive deficit, memory/attention problems, and sleep disorders (Pilotto et al., 2021). Those with cognitive deficits had had worse respiratory COVID-19 symptoms and had been in the hospital for longer than those who were cognitively normal.
To put these disparate bits and pieces of data together, prospective studies are now being set up to track neurological consequences of the disease. The National Institute of Neurological Disorders and Stroke (NINDS) COVID-19 Neuro Databank/Biobank (NeuroCOVID) will collate information on neurological symptoms, complications, and outcomes, as well as SARS-CoV-2’s effect on pre-existing neurological conditions. Led by Andrea Troxel and Eva Petkova at NYU Langone Health, New York City, NeuroCOVID will also collect biological samples for analysis (press release).
This effort joins other databanks collecting information on COVID-19 neurological symptoms/outcomes and biospecimens, including the Global Consortium Studies of Neurological Dysfunction in COVID-19 (GCS-NeuroCOVID), the German Infectious Disease Society’s Lean European Open Survey on SARS-CoV-2 infected patients (LEOSS, and Brain Infections Global), and the University of Liverpool's COVID-19 Neuro Network (Helbok et al., 2020).
The European Academy of Neurology created the Neuro-COVID Registry Consortium [ENERGY], a prospective registry to track neurological symptoms and their outcomes in confirmed COVID-19 cases at six and 12 months (Beghi et al., 2020). At least 254 centers from 69 countries and four continents have requested to join the study.
The Scary Question
Could these patients be at higher risk for subsequent dementia, including AD? The Consortium for Chronic Neuropsychiatric Sequelae of SARS-CoV-2, led by Sudha Seshadri, University of Texas Health Science Center, San Antonio, and sponsored by the Alzheimer’s Association and World Health Organization, hopes to have some answers within a year or two (de Erausquin et al., 2021). Researchers from more than 30 countries are recruiting people who were hospitalized with COVID-19, and will study them at six, nine, and 18 months after discharge. People currently involved in other international research studies will serve as controls.
The researchers aim to enroll 1,000 people per site for a total of up to 40,000 participants. They will collect fluid samples and brain scans and examine factors such as health disparities and cultural differences. “The exact measures will vary somewhat from country to country based on availability and funding,” Gabriel de Erausquin, a consortium investigator at the UT Health Science Center, told Alzforum. “Overall, we intend to collect brain MRI, CSF and plasma for biomarker analysis, and DNA for genetic studies.” CSF biomarkers include Aβ42, Aβ42/40, phospho-tau and total tau.
“Previous data strongly reiterate the need to carefully study the long-term implications and consequences of COVID-19 infection for risk of AD and related dementias in large sample sizes with replication across many countries,” said Seshadri.—Chelsea Weidman Burke
References
News Citations
- APOE Tied to Increased Susceptibility to SARS-CoV-2
- Herpes Simplex Virus Triggers Amyloidosis in 3D Neural Cultures
Paper Citations
- Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Kazmi SA, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas JL, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021 Mar 1;218(3) PubMed.
- Wang C, Zhang M, Garcia G Jr, Tian E, Cui Q, Chen X, Sun G, Wang J, Arumugaswami V, Shi Y. ApoE-Isoform-Dependent SARS-CoV-2 Neurotropism and Cellular Response. Cell Stem Cell. 2021 Feb 4;28(2):331-342.e5. Epub 2021 Jan 4 PubMed.
- Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu YF, Gong HR, Lee AC, Zou Z, Yau T, Wu W, Hung IF, Chan JF, Yuen KY, Huang JD. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020 Oct;30(10):928-931. Epub 2020 Aug 4 PubMed.
- Jacob F, Pather SR, Huang WK, Zhang F, Wong SZ, Zhou H, Cubitt B, Fan W, Chen CZ, Xu M, Pradhan M, Zhang DY, Zheng W, Bang AG, Song H, Carlos de la Torre J, Ming GL. Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell. 2020 Dec 3;27(6):937-950.e9. Epub 2020 Sep 21 PubMed.
- Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, James LC, Lancaster MA. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell. 2020 Dec 3;27(6):951-961.e5. Epub 2020 Oct 13 PubMed.
- Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, Mariappan A, Goureau O, Gruell H, Walker A, Andrée M, Hauka S, Houwaart T, Dilthey A, Wohlgemuth K, Omran H, Klein F, Wieczorek D, Adams O, Timm J, Korth C, Schaal H, Gopalakrishnan J. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020 Oct 15;39(20):e106230. Epub 2020 Sep 23 PubMed.
- Le Guennec L, Devianne J, Jalin L, Cao A, Galanaud D, Navarro V, Boutolleau D, Rohaut B, Weiss N, Demeret S. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020 Aug;61(8):e90-e94. Epub 2020 Jul 23 PubMed.
- Politi LS, Salsano E, Grimaldi M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020 Aug 1;77(8):1028-1029. PubMed.
- Ismail II, Gad KA. Absent Blood Oxygen Level-Dependent Functional Magnetic Resonance Imaging Activation of the Orbitofrontal Cortex in a Patient With Persistent Cacosmia and Cacogeusia After COVID-19 Infection. JAMA Neurol. 2021 Jan 22; PubMed.
- Coolen T, Lolli V, Sadeghi N, Rovai A, Trotta N, Taccone FS, Creteur J, Henrard S, Goffard JC, Dewitte O, Naeije G, Goldman S, De Tiège X. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020 Oct 6;95(14):e2016-e2027. Epub 2020 Jun 16 PubMed.
- Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, Masliah E, Horkayne-Szakaly I, Jones R, Stram MN, Moncur J, Hefti M, Folkerth RD, Nath A. Microvascular Injury in the Brains of Patients with Covid-19. N Engl J Med. 2020 Dec 30; PubMed.
- Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021 Feb;24(2):168-175. Epub 2020 Nov 30 PubMed.
- Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF Jr, Sabeti P. Neuropathological Features of Covid-19. N Engl J Med. 2020 Sep 3;383(10):989-992. Epub 2020 Jun 12 PubMed.
- Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, Li Z, Deng P, Zhang J, Zhong N, Ding Y, Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005 Oct 15;41(8):1089-96. Epub 2005 Sep 12 PubMed.
- McCray PB Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007 Jan;81(2):813-21. Epub 2006 Nov 1 PubMed.
- Kumari P, Rothan HA, Natekar JP, Stone S, Pathak H, Strate PG, Arora K, Brinton MA, Kumar M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses. 2021 Jan 19;13(1) PubMed.
- Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2020 Dec 16; PubMed.
- Virhammar J, Nääs A, Fällmar D, Cunningham JL, Klang A, Ashton NJ, Jackmann S, Westman G, Frithiof R, Blennow K, Zetterberg H, Kumlien E, Rostami E. Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur J Neurol. 2020 Dec 28; PubMed.
- DeKosky ST, Kochanek PM, Valadka AB, Clark RS, Chou SH, Au AK, Horvat C, Jha RM, Mannix R, Wisniewski SR, Wintermark M, Rowell SE, Welch RD, Lewis L, House S, Tanzi RE, Smith DR, Vittor AY, Denslow ND, Davis MD, Glushakova OY, Hayes RL. Blood Biomarkers for Detection of Brain Injury in COVID-19 Patients. J Neurotrauma. 2021 Jan 1;38(1):1-43. Epub 2020 Nov 11 PubMed.
- Edén A, Kanberg N, Gostner J, Fuchs D, Hagberg L, Andersson LM, Lindh M, Price RW, Zetterberg H, Gisslén M. CSF Biomarkers in Patients With COVID-19 and Neurologic Symptoms: A Case Series. Neurology. 2021 Jan 12;96(2):e294-e300. Epub 2020 Oct 1 PubMed.
- Garcia MA, Barreras PV, Lewis A, Pinilla G, Sokoll LJ, Kickler T, Mostafa H, Caturegli M, Moghekar A, Fitzgerald KC, Pardo CA. Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation. medRxiv. 2021 Jan 12; PubMed.
- Kanberg N, Ashton NJ, Andersson LM, Yilmaz A, Lindh M, Nilsson S, Price RW, Blennow K, Zetterberg H, Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020 Sep 22;95(12):e1754-e1759. Epub 2020 Jun 16 PubMed.
- Sutter R, Hert L, De Marchis GM, Twerenbold R, Kappos L, Naegelin Y, Kuster GM, Benkert P, Jost J, Maceski AM, Rüegg S, Siegemund M, Leppert D, Tschudin-Sutter S, Kuhle J. Serum Neurofilament Light Chain Levels in the Intensive Care Unit: Comparison between Severely Ill Patients with and without Coronavirus Disease 2019. Ann Neurol. 2020 Dec 30; PubMed.
- Pilotto A, Cristillo V, Piccinelli SC, Zoppi N, Bonzi G, Sattin D, Schiavolin S, Raggi A, Canale A, Gipponi S, Libri I, Frigerio M, Bezzi M, Leonardi M, Padovani A. COVID-19 severity impacts on long-term neurological manifestation after hospitalisation. MedRxiv. Posted January 02, 2021. doi.org/10.1101/2020.12.27.20248903 medRxiv.
- Helbok R, Chou SH, Beghi E, Mainali S, Frontera J, Robertson C, Fink E, Schober M, Moro E, McNett M, Bassetti CL, GCS-NeuroCOVID consortium, EAN COVID task force. NeuroCOVID: it's time to join forces globally. Lancet Neurol. 2020 Oct;19(10):805-806. Epub 2020 Sep 16 PubMed.
- Beghi E, Helbok R, Crean M, Chou SH, McNett M, Moro E, Bassetti C, EAN Neuro-COVID Task Force. The European Academy of Neurology COVID-19 registry (ENERGY): an international instrument for surveillance of neurological complications in patients with COVID-19. Eur J Neurol. 2020 Nov 21; PubMed.
- de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S, CNS SARS-CoV-2 Consortium. The chronic neuropsychiatric sequelae of COVID-19: The need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 2021 Jun;17(6):1056-1065. Epub 2021 Jan 5 PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S, CNS SARS-CoV-2 Consortium. The chronic neuropsychiatric sequelae of COVID-19: The need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 2021 Jun;17(6):1056-1065. Epub 2021 Jan 5 PubMed.
- Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Kazmi SA, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas JL, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021 Mar 1;218(3) PubMed.
- Wang C, Zhang M, Garcia G Jr, Tian E, Cui Q, Chen X, Sun G, Wang J, Arumugaswami V, Shi Y. ApoE-Isoform-Dependent SARS-CoV-2 Neurotropism and Cellular Response. Cell Stem Cell. 2021 Feb 4;28(2):331-342.e5. Epub 2021 Jan 4 PubMed.
- Ismail II, Gad KA. Absent Blood Oxygen Level-Dependent Functional Magnetic Resonance Imaging Activation of the Orbitofrontal Cortex in a Patient With Persistent Cacosmia and Cacogeusia After COVID-19 Infection. JAMA Neurol. 2021 Jan 22; PubMed.
- Kumari P, Rothan HA, Natekar JP, Stone S, Pathak H, Strate PG, Arora K, Brinton MA, Kumar M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses. 2021 Jan 19;13(1) PubMed.
- Edén A, Kanberg N, Gostner J, Fuchs D, Hagberg L, Andersson LM, Lindh M, Price RW, Zetterberg H, Gisslén M. CSF Biomarkers in Patients With COVID-19 and Neurologic Symptoms: A Case Series. Neurology. 2021 Jan 12;96(2):e294-e300. Epub 2020 Oct 1 PubMed.
- Garcia MA, Barreras PV, Lewis A, Pinilla G, Sokoll LJ, Kickler T, Mostafa H, Caturegli M, Moghekar A, Fitzgerald KC, Pardo CA. Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation. medRxiv. 2021 Jan 12; PubMed.
- Pilotto A, Cristillo V, Piccinelli SC, Zoppi N, Bonzi G, Sattin D, Schiavolin S, Raggi A, Canale A, Gipponi S, Libri I, Frigerio M, Bezzi M, Leonardi M, Padovani A. COVID-19 severity impacts on long-term neurological manifestation after hospitalisation. MedRxiv. Posted January 02, 2021. doi.org/10.1101/2020.12.27.20248903 medRxiv.
Annotate
To make an annotation you must Login or Register.

Comments
OHSU
Our findings show that the SARS-CoV-2 viral spike protein S1, which is required for cellular uptake of virus, can enter the mouse brain following intravenous or intranasal administration. This might relate to the fact that people with COVID-19 are often suffering cognitive effects, such as brain fog, and fatigue.
This finding is also important because binding proteins such as S1 can detach from the virus and themselves cause toxicity in patients following COVID-19 infection. The S1 protein in SARS-CoV-2 and the gp 120 protein in HIV-1 might function similarly. They are glycoproteins—proteins that have a lot of sugars on them, hallmarks of proteins that bind to other receptors. Both proteins function as the arms and hands for their viruses by grabbing onto other glycoproteins on the cell surface. Both cross the blood-brain barrier and S1, like gp120, is likely toxic to brain tissues. We (Bill Banks and myself) worked with recombinant gp 120 as part of previous mouse studies.…More
The breathing problems seen following COVID-19 infection might not only relate to infection in the lungs but also to respiratory centers of the brain following viral uptake in the brain. We find that transport of S1 is faster in the olfactory bulb and kidney of males than females. This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes. Our findings that wild-type mice, which do not express the human ACE2 receptor, can be used in kinetics studies of the S1 protein and probably SARS-CoV-2 suggest that the assumption that human ACE2 must be used for these kind of studies in mice, is incorrect. These results also support the use of recombinant proteins or virus-like proteins to increase our knowledge about the effects of SARS-CoV-2 proteins on the brain, rather than active replicating viruses, which require additional biosafety precautions and animal facilities.
References:
Raber J, Toggas SM, Lee S, Bloom FE, Epstein CJ, Mucke L. Central nervous system expression of HIV-1 Gp120 activates the hypothalamic-pituitary-adrenal axis: evidence for involvement of NMDA receptors and nitric oxide synthase. Virology. 1996 Dec 15;226(2):362-73. PubMed.
Banks WA, Kastin AJ, Akerstrom V. HIV-1 protein gp120 crosses the blood-brain barrier: role of adsorptive endocytosis. Life Sci. 1997;61(9):PL119-25. PubMed.
These findings are quite consistent with reports, espoused by many eminent researchers, and central to our stance on www.ALZgerm.org, that many viruses, bacteria, and parasites can enter the brain, either temporarily or long-term. They may live quietly, trigger inflammation, or directly damage brain cells. And so, COVID joins herpes simplex, spirochetes, toxoplasma, chlamydia, mycobacteria, and prions as a possible future trigger of AD.
We also recall that after chickenpox, the neurotropic herpes zoster virus becomes latent for decades until emerging as shingles. The neurotrophic spirochetes of syphilis, and perhaps borrelia, may damage the brain years after initial infection and then latency. As I warned in comments on Alzforum April 1, 2020, COVID infections may disturb, visibly or not, future neurologic findings and outcomes in cohorts of seniors being followed longitudinally in dementia studies.…More
Make a Comment
To make a comment you must login or register.