Gantenerumab Mystery: How Did It Lose Potency in Phase 3?
Quick Links
At first blush, recent Phase 3 trial results from the anti-amyloid antibodies gantenerumab and lecanemab seem to be a study in opposites, one negative and one positive. At the 15th Clinical Trials on Alzheimer’s Disease conference, held November 29 to December 2 in San Francisco, however, scientists said both programs together paint one and the same picture, of plaque needing to be completely cleared before the brain can—ever so slowly—respond.
- In the negative Graduate trials, gantenerumab removed half as much plaque as expected.
- On clinical endpoints, trends favored drug.
- Roche is examining pharmacokinetic data to learn what went wrong.
This is because data from the two Phase 3 Graduate studies of Roche and Genentech’s gantenerumab showed that the antibody took but weak jabs at its target, clearing half as much plaque as expected during the trial’s more than two years of dosing. Far fewer participants became amyloid-negative as per PET than in the more fortunate Clarity trial of lecanemab. In Graduate, clinical measures all trended in the right direction, but the small changes fell short of statistical significance. Participants who dropped below the threshold for brain-wide amyloid positivity fared best.
It is unclear what went wrong with plaque clearance; Roche is currently analyzing pharmacokinetic data in search of answers. In the meantime, the company has halted its gantenerumab trials, though it continues to make the drug available to the Dominantly Inherited Alzheimer's Disease Trials Unit.

A Signal Is Not Enough. In the pooled Graduate data, gantenerumab nudged down decline about 8 percent, with nominal statistical significance. [Courtesy of Roche.]
Disappointment about the result was palpable everywhere at CTAD, as was praise for the quality of the clinical research program. “These were wonderful studies with extreme scientific rigor,” said Lefkos Middleton of Imperial College London. Randall Bateman of Washington University, St. Louis, who presented the results, emphasized the contribution even these negative findings can make. “This is an incredibly valuable dataset that will steer the field toward achieving larger-magnitude effects,” Bateman said in San Francisco. Roche also drew praise for sharing data publicly so soon—two weeks—after the data blind was broken.
Also at CTAD, plasma biomarker data from the negative Phase 3 trial of crenezumab were shown. As with previously reported cerebrospinal fluid markers, measures of tau pathology, inflammation, and neurodegeneration edged toward normal, but did not reach statistical significance. Clinical development of this drug is now over. [Correction posted January 19, 2023: According to Roche, evaluation of crenezumab in the API trial is concluding. However, according to AC Immune, the overall crenezumab development program has not been terminated].
Trends, But Nothing to Write Home About
Roche scientists selected gantenerumab’s Phase 3 dose based on open-label extension studies of their Scarlet Road and Marguerite Road trials. There, when given as a monthly subcutaneous injection of 1,200 mg, gantenerumab completely cleared plaque in half of participants at two years, and in 80 percent at three years (Dec 2017 conference news; Dec 2019 conference news).
The Graduate trials used a slightly lower dose, 1,020 mg given as two 510 mg injections every two weeks for 27 months. Doody noted that Roche switched to this split dosing to lower the volume that needed to be injected each time, and to make the shots less uncomfortable for patients. Subcutaneous antibody is delivered via syringe, usually into the abdomen, and can cause local reactions such as redness and swelling. In a poster at CTAD, Beate Bittner at Roche reported that participants found the pain from SC injections tolerable, fading within five minutes. Some OLE participants also received split doses, and PK analysis prior to Graduate had shown that it produced the same gantenerumab plasma levels the larger volumes did, Doody said.
Participants were titrated up to this level over nine months, meaning they got the target dose for 18 months, the same duration as the lecanemab Clarity trial.
The two trials, which ran from 2018 to 2022, enrolled 1,965 people from 30 countries, half of whom received gantenerumab. Participants were an average of 72 years old, with about 17 percent Hispanic or Latino, 12 percent Asian, and 3 percent American Indian or Alaska Native. Just over half the cohort, 55 percent, was diagnosed with mild cognitive impairment at baseline; the rest had mild dementia. The global CDR scores were slightly imbalanced, with three-fourths of people in the placebo groups having a CDR of 0.5, compared to two-thirds of people on gantenerumab. The remainder of both groups were more impaired, with a CDR of 1. Some researchers at CTAD wondered whether the greater impairment in the treatment group might have affected the results. Even if it did, the small difference is unlikely to explain why gantenerumab fell so far short of expectations, Doody told Alzforum.
As shown in the topline results, gantenerumab failed to slow decline on the primary outcome measure, the CDR-SB (Nov 2022 news). Treatment and placebo groups did diverge slightly, with people on gantenerumab posting a 0.31 and 0.19 point better score than those on placebo in Graduate 1 and 2, respectively, but this was statistically insignificant. In a prespecified pooled analysis, people on gantenerumab did 0.26 points better than those on placebo overall. This represented an 8 percent slowing of decline, which was nominally significant at p=0.04.
Results on secondary endpoints were similar, with trends favoring drug. Gantenerumab held back decline by about 14 percent on the ADAS-Cog13, 12 percent on the Functional Activities Questionnaire, and 9 percent on the ADCS Activities of Daily Living. None of this was statistically significant, though the ADAS-Cog13 and FAQ came close, averaging p=0.04.

Too Little Too Late. In Graduate 1 (left) and 2 (right), gantenerumab (blue) cleared plaque half as fast as projected over two years of treatment. [Courtesy of Roche.]
Weak Effect on Underlying Pathology What about amyloid removal? In the amyloid PET substudy of 383 people, gantenerumab cut plaque by about 23 centiloids after one year, and 53 centiloids after two years. Based on the prior OLE data, Roche scientists had expected reductions of 42 and 71 centiloids at one and two years, respectively. As a consequence, only 27 percent of people taking gantenerumab became amyloid-negative by two years, in contrast to half of people in the OLE, and two-thirds of people in the positive Clarity trial of lecanemab.
To explore whether plaque clearance affected the results, researchers compared CDR-SB scores in people who dropped below the positivity threshold of 24 centiloids and those who did not. The latter slipped three points on the CDR-SB over the course of the trial, identical to the placebo group, while the former slid two points, a third less decline. The results from this exploratory post hoc analysis add to the field’s growing sense that complete plaque clearance is key for realizing a cognitive benefit.
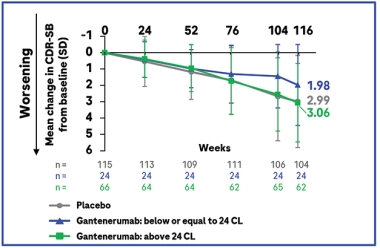
Clearance Matters. When participants were stratified by how much of their amyloid was removed, those with complete plaque clearance (blue) notched a cognitive benefit, unlike those without (green). Placebo group in gray. [Courtesy of Roche.]
The brain edema known as ARIA-E showed up as expected, in 22 percent of participants. This puts gantenerumab between aducanumab’s 33 percent and lecanemab’s 12 percent for this side effect. Five percent of people on gantenerumab developed symptoms from ARIA; 1 percent had serious symptoms. As with other anti-amyloid antibodies, ARIA-E was worse in APOE4 carriers, who made up two-thirds of the cohort. It occurred in 48 percent of E4 homozygotes, 24 percent of heterozygotes, and 12 percent of noncarriers.
Pierre Tariot of the Banner Alzheimer Institute in Phoenix asked why ARIA rates were similar to those in previous gantenerumab trials, even though amyloid removal was lower. Bateman noted that ARIA rose toward the end of the trial, when plaque removal sped up.
Cerebrospinal fluid biomarkers changed in the expected direction on gantenerumab. P-tau181 dropped by 24 percent; total tau, 18 percent. Neurogranin, which reflects synaptic loss, fell by 22 percent. All markers stayed relatively flat in the placebo group, and the differences were statistically significant. As with other anti-amyloid antibodies, the neurodegeneration marker NfL continued to rise on gantenerumab, but more slowly, by 11 percent rather than the 20 percent seen in the placebo group.
Two Decades of Development. The Graduate studies grew out of years of experiments to refine the antibody’s dosing and administration. [Courtesy of Roche.]
What to Make of It All?
Scientists at CTAD debated whether the result reflects a problem with how much gantenerumab was given, how it was given, or how the antibody was formulated. Robert Vassar of Northwestern University, Chicago, asked whether the subcutaneous administration was as reliable as intravenous dosing, which other antibodies still use. Most companies in the field are attempting to move to injection under the skin—is this a setback that should give them pause?
Doody said the company’s prior studies indicated that gantenerumab did reach adequate exposure via this route. At CTAD, Bittner presented pharmacokinetic analyses showing similar plasma concentrations of gantenerumab after IV or SC administration. All gantenerumab dosing has been subcutaneous since 2008, inspiring other anti-amyloid antibody programs to follow in hopes of lowering cost, relying less on infusion centers, and making the whole procedure more convenient for patients. In a Roche study of at-home administration, caregivers were able to dose patients using an auto-injector; 93 percent of caregivers felt confident doing so.
Others thought the antibody itself may have lost potency since earlier trials. Robert Przybelski of the University of Wisconsin, Madison, previously worked for Baxter Healthcare Corporation. Przybelski noted that when biologics drug production is scaled up for large, global trials, changes in the manufacturing methodology can reduce its effectiveness. Monoclonal antibodies are produced by cell lines grown in large bioreactors, and are harvested and purified in batches. Przybelski asked whether logs of batch activity testing might be consulted to probe for differences.
Doody acknowledged that Roche changed the cell line it used when production scaled up for Phase 3. This drew little notice at the time, but now has raised eyebrows as a possible culprit. Roche is looking into whether changes in formulation or production had an effect, Doody said.
Stephen Salloway of Butler Hospital in Providence, Rhode Island, brought up another angle, pointing out that the Graduate and Clarity populations were not the same. The Graduate participants started out with more amyloid, averaging 95 centiloids at baseline rather than 76. The population was also more impaired, with an average MMSE of 23 and CDR-SB of 3.7, and with 45 percent of participants having mild dementia. For lecanemab's Clarity trial, these numbers were 26, 3.2, and 38 percent, respectively. Would an earlier-stage population have gained more from treatment? Supporting this, a prespecified analysis by disease stage indicated that participants with MCI fared better on gantenerumab than those with mild dementia, losing 0.20 fewer points on the CDR-SB.
Given the trends in the outcome data all pointing in the same direction, David Morgan of Michigan State University, Grand Rapids, speculated that a longer trial might yet have demonstrated a benefit. Christopher van Dyck of Yale School of Medicine in New Haven, Connecticut, suggested exploring higher doses or faster titration.
Doody grasped at no straws, however. Although the Graduate results were disappointing, she said, they were very clear. Efficacy was insufficient. As a result, she said, Roche has halted its OLE studies of gantenerumab, as well as the at-home administration study. Ditto for the Skyline secondary prevention trial (Mar 2022 news). This latter news sparked dismay in the audience. Suzanne Hendrix of Pentara Corporation, Salt Lake City, questioned this decision, given the apparent higher benefits at earlier disease stages. So did others. Alas, Doody said the negative Graduate findings had shifted the risk/benefit ratio of gantenerumab.
While Graduate was still ongoing, the Dominantly Inherited Alzheimer Network had chosen gantenerumab for a primary prevention trial. This trial has been engaging mutation carriers older than 18 in a cognitive run-in phase to gather biomarker data and improve statistical power before a drug had even been chosen. DIAN-TU discussed Graduate's topline results with its participating families, who decided they wanted to continue with the plan. Indeed, the primary prevention trial consented its first patient during the CTAD conference, Eric McDade of Washington University, St. Louis, told Alzforum. After the conference, however, DIAN-TU leaders made the decision to switch from gantenerumab, because there might not be enough antibody available to finish the trial. This trial will give a study drug for four years during its blinded phase and then for four more years in open-label extension. DIAN-TU will continue to enroll participants in the cognitive run-in while choosing a new drug (see announcement).
DIAN-TU is still dosing with gantenerumab in the OLE of its secondary prevention study (Apr 2020 conference news; Jun 2021 news). In this program, DIAN uses a three times higher dose than Graduate did, spread out over several injections. Doody noted that the higher dosing might overcome any loss of potency in the antibody. Roche is currently making gantenerumab available to this OLE, and is in discussions with DIAN-TU regarding next steps.
Doody emphasized that despite the negative results for this formulation of gantenerumab, Roche is pursuing alternatives. One is its brain-shuttle delivery program. Both arms of this antibody contain the amyloid-binding portion of gantenerumab and it is attached to the transferrin receptor shuttle molecule by a chain. The receptor ferries the antibody into the brain, achieving 10- to 20-fold higher brain concentration than peripheral administration (Mar 2021 conference news; Dec 2021 conference news). Currently in a Phase 1b/2a study, the shuttle antibody, dubbed trontinemab, is being developed separately from gantenerumab, and may well have different effects due to its broader distribution through the brain, Doody noted. “The field needs ways to get more antibody into the brain,” she told Alzforum.
Farewell Crenezumab
Crenezumab is IgG4 antibody that targets Aβ oligomers and monomers but does not clear plaque. It missed primary and secondary endpoints in the API Colombian prevention study, though trends on nearly all endpoints favored drug (Jun 2022 news; Aug 2022 conference news). Roche currently has no plans for testing it further.
In San Francisco, Eric Reiman of Banner presented plasma data. It was collected from all participants over several years, but analyzed only recently in one batch at a single lab. At baseline, cognitively unimpaired carriers of the E280A Paisa mutation in presenilin 1 had higher p-tau181 and p-tau217 than the 83 noncarriers, as expected. Their plasma Aβ42/40 ratio was higher than in noncarriers, reflecting overproduction of the toxic form of Aβ. In addition, carriers had more of the inflammation marker GFAP and the neurodegeneration marker NfL than did noncarriers.
Over time, all markers except Aβ42/40 rose in the 84 carriers on placebo. The inflammatory marker sTREM2, while not elevated at baseline, also rose in carriers over time. Meanwhile, the Aβ ratio dropped, indicating Aβ42 sequestration into plaques. In the 85 mutation carriers taking crenezumab, plasma Aβ42 rose significantly, likely as a result of crenezumab binding it and slowing its clearance. All other markers trended back toward normal, but the changes were not statistically significant. These markers are measured in pg/mL.
Separate subgroup analyses of those carriers who were still amyloid negative versus already amyloid-positive at baseline are underway, Reiman said. In the meantime, API is closing out the study, finishing final visits with participants now. Other drugs will be evaluated in this population in the future, Reiman noted.—Madolyn Bowman Rogers
References
Therapeutics Citations
News Citations
- High-Dose Gantenerumab Lowers Plaque Load
- Amyloid Clearance: Check. Cognitive Benefit: Um … Maybe.
- Gantenerumab Falls Short in Phase 3
- Gantenerumab Prevention Trial in Sporadic Alzheimer's Begins
- In DIAN-TU, Gantenerumab Brings Down Tau. By a Lot. Open Extension Planned
- Paper Alert: DIAN-TU Solanezumab and Gantenerumab Data Published
- Shuttle Unloads More Gantenerumab Into the Brain
- Brain Shuttle Could Halve Amount of Gantenerumab Needed
- API Colombian Trial of Crenezumab Missed Primary Endpoints
- Crenezumab Secondaries Negative; Gantenerumab OLE Hints at Efficacy
Mutations Citations
Other Citations
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.