Crenezumab Secondaries Negative; Gantenerumab OLE Hints at Efficacy
Quick Links
While the Alzheimer’s field awaits pivotal results from three Phase 3 trials of anti-amyloid antibodies, data on this class of therapeutics continue to trickle in. At the Alzheimer’s Association International Conference held July 31-August 4 virtually and in San Diego, California, researchers expanded on previously reported negative topline results from the Alzheimer Prevention Initiative’s trial of crenezumab in Colombia. Crenezumab, made by Roche/Genentech, differs from other anti-amyloid antibodies in late-stage trials in that it targets Aβ monomers and oligomers rather than fibrillar forms. As had been the case with memory and cognitive co-primary endpoints, all secondary clinical and biomarker outcomes favored the drug, but the difference did not reach statistical significance. Dose-response curves, subgroup analyses, and plasma biomarkers are still being analyzed, with the goal to inform future studies in the Colombian autosomal-dominant AD kindred.
- In the API Colombian trial, all endpoints fell short of significance but favored crenezumab.
- The trial lacked power to detect a clinical effect.
- Gantenerumab may have slowed cognitive decline in open-label extension studies.
Meanwhile, the Phase 3 GRADUATE trials of Roche’s fibrillar-targeting antibody, gantenerumab, are expected to read out this fall. At AAIC, scientists presented a smidgen of new data from the open-label extension studies of the older, and negative, SCarlet RoAD and Marguerite RoAD trials, showing plasma biomarker effects, plus a potential clinical benefit when compared to the expected rate of cognitive decline in matched controls. Janice Smith leads the gantenerumab program. She noted that the drug has been in development for two decades, with more than 2,600 patient-years of testing. Both the gantenerumab and crenezumab programs represent an enormous investment of time and resources.

Consistent Trend? In the API Colombian trial, clinical and biomarker endpoints all favored crenezumab, though none reached statistical significance. [Courtesy of Roche.]
Crenezumab Data: Stepping-Stone to New Trials?
The API Colombian trial of crenezumab pioneered prevention studies for disease-modifying AD drugs, demonstrating it was possible to run such trials. It enrolled 169 people who carried the E280A Paisa mutation in presenilin 1; 85 of them received subcutaneous crenezumab, 84 placebo. The trial also included 83 noncarriers, all of whom received placebo. At baseline, almost half of carriers were amyloid-negative (Aug 2019 conference news). The study ran for eight years, with an astonishing 94 percent retention rate. At AAIC, Pierre Tariot of the Banner Alzheimer Institute in Phoenix quipped that it was a “huge small trial.”
Roche previously reported the trial was negative on both primaries, the Free and Cued Selective Reminding Test (FCSRT) and the API cognitive composite (Jun 2022 news). In San Diego, Tariot put numbers to this. On average, people on crenezumab declined 20 percent more slowly on the FCSRT than did carriers on placebo; on the composite, they declined 23 percent more slowly. On secondary clinical endpoints, the crenezumab group declined 9 percent more slowly on the CDR-SB, 8 percent on the CDR, and 44 percent more slowly on RBANS, a neuropsychological composite. That said, variability within the treatment groups was large, and none of the differences were statistically significant.
Progression to mild cognitive impairment or dementia also appeared to slow down a tad on drug, Tariot reported. Progression curves were identical for the first four years of the trial, and then separated, with the treatment group progressing 21 percent more slowly after eight years. As with the other endpoints, this difference was not statistically significant, and Tariot cautioned that the number of participants at later timepoints was quite small.
Biomarkers followed a similar pattern. FDG PET, which measures the brain’s glucose use, weakened 18 percent less on drug than placebo. MRI scans showed 8 percent less whole brain shrinkage on drug than placebo; regional MRI findings are still being analyzed. Tau PET was added to this trial late and done for only 83 mutation carriers. The PET signal rose 51 percent less in those on drug than placebo. Cerebrospinal fluid was drawn from about half of participants. CSF p-tau181, total tau, and NfL worsened 37, 29, and 18 percent less on drug than on placebo, respectively. For all these imaging and fluid biomarkers, the variability within each treatment group was large. None of the findings were statistically significant.
One exception was CSF Aβ42 and Aβ40 concentrations. Aβ42 stabilized on drug, but declined in controls; Aβ40 rose on drug and dropped in controls. Both changes were statistically significant. Eric Reiman at Banner noted that the uptick in these peptides may be due to slower clearance of antibody-bound Aβ from brain and CSF, indicating that crenezumab interacts with its target. The drop in Aβ40 in placebo controls was unexpected; Reiman speculated this could reflect its sequestration into vascular plaques.
Other CSF markers, including α-synuclein, neurogranin, and numerous inflammatory proteins, remain to be analyzed. Also ongoing is analysis of oligomeric Aβ, which will reveal how effective crenezumab was at engaging its target. The researchers did not present any data on how plasma and CSF concentrations of crenezumab differed, which would indicate how much drug reached the central nervous system.
API scientists showed only one subgroup analysis. They compared decline on the two primary endpoints in mutation carriers who were amyloid-positive versus amyloid-negative at baseline. This analysis hinted at a bigger benefit in the amyloid-negative group, but again, this was statistically insignificant. Yet to come are dose-exposure data and “spaghetti plots” showing individual trajectories. The large variation within small treatment groups could make personalized trajectories on all outcomes and biomarkers measured in a given person over time, from baseline onward, particularly revealing.
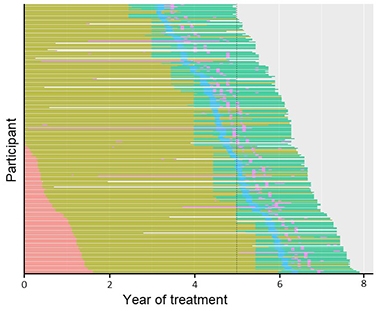
Dose Escalation. Dosing in the API Colombian study increased over time from 300 mg subcutaneous (pink) to 720 SC (olive) to 60 mg/kg intravenous (aqua); all participants missed doses due to the COVID shutdown (blue), with some missing other doses (purple). [Courtesy of Roche.]
Reiman noted several limitations. Partway through the eight-year trial, the field realized that antibodies needed to be given at much greater doses than originally thought. The researchers boosted the dosage twice and switched from subcutaneous injections to intravenous. At study start people received 300 mg crenezumab per month subcutaneously, and by the end, 60 mg/kg per month intravenously, which Roche researchers noted was an increase of more than sevenfold. Alas, participants were only on this highest dose for about two years. This means the drug may not have had enough time at its effective dose to generate a benefit. To complicate matters further, right after switching to the highest dose, COVID pandemic shutdowns caused participants to miss on average four doses, noted Rachelle Doody at Roche. Even so, over the whole trial, 88 percent of scheduled doses were delivered.
The trial had less statistical power than anticipated. Partly, this was because the cognitive composite was developed to detect decline in the larger observational cohort of the Colombian kindred. However, mutation carriers who enrolled in the trial were on average four years younger than the observational cohort, and twice as likely to be amyloid-negative. This meant the composite lacked sensitivity to detect early cognitive change in the participants.
Sensitivity analyses found that the FCSRT was a better measure. Tariot noted that the FCSRT had 63 percent power to detect a 30 percent slowing of decline in this trial, while the composite had only 6 percent. Future trials in this kindred should therefore use the FCSRT, and might need to enroll twice as many carriers, Tariot believes. While researchers decide on the appropriate drug and design for the next trial in this population, all mutation carriers in the current trial will receive crenezumab in an open-label extension, while noncarriers continue to receive placebo.
Gantenerumab Open-Label Data Hints at Cognitive Benefit
Like crenezumab, gantenerumab’s dosage was also boosted during the course of its trials. After two negative Phase 3s, SCarlet RoAD and Marguerite RoAD, Roche upped the amount they gave in the open-label extensions (OLE) fivefold, to 1,200 mg given subcutaneously. After three years at this higher dose, 80 percent of participants had fallen below the threshold for amyloid positivity (Dec 2017 conference news; Dec 2019 conference news).
At AAIC, Tobias Bittner at Roche reported plasma biomarker findings from these OLEs. Over three years, plasma Aβ40 and Aβ42 concentrations each nearly doubled, while the Aβ42/40 ratio nudged up by about 10 percent. Meanwhile, plasma p-tau217 and p-tau181 edged downward by about 10 percent over three years. The findings complement results from other studies, in which gantenerumab reduced total tau and p-tau181 in CSF and slowed the rise of the neurodegeneration marker NfL (Jun 2021 news).
Because OLEs have no placebo control, clinical effects of the treatment cannot be directly evaluated. To estimate them, statistician Paul Delmar at Roche used ADNI observational data as an external control. In his analysis, he included 147 OLE participants with mild cognitive impairment or mild AD dementia, defined as an MMSE of 18 or higher, CDR of 1 or lower, CDR-SB of 5.5 or lower, and ADAS-Cog13 of 38 or lower. He screened 1,530 ADNI participants to find 430 that matched the OLE cohort on multiple criteria, such as sex, age, APOE genotype, education, and cognitive scores. All participants were amyloid-positive and 50 or older. The ADNI participants who were most similar to RoAD participants were given more weight in the statistical analysis.
On the CDR-SB, the cohorts started to diverge at two years, with the RoAD OLE cohort declining 24 percent less than ADNI controls; by three years, this difference grew to 39 percent. Results were similar for the ADAS-Cog13 and the MMSE, with the OLE cohort declining 36 and 19 percent less than ADNI participants at three years, respectively. The findings were statistically significant. “These results provide context and support the potential clinical relevance of the biomarker findings,” Delmar said.
Delmar also reported that the cognitive benefit correlated with amyloid removal. In people with the most plaque clearance, CDR-SB changed little over three years, whereas in those with the least clearance, CDR-SB rose about 4 points. The correlation was weak, at r=0.19. However, in the 34 OLE participants with four-year data, correlation strengthened somewhat to r=0.44. “This speaks to the importance of considering the long-term effects of the drug,” Delmar said. Smith agreed, noting that the clinical benefit of gantenerumab appears to increase over time.
Other talks at AAIC attempted to model the long-term effects of anti-amyloid antibody treatment. Overall, they likewise concluded that progression may slow as treatment continues (see related conference story).—Madolyn Bowman Rogers
References
Therapeutics Citations
Mutations Citations
News Citations
- Crenezumab Update: Baseline Data from Colombian Prevention Trial
- API Colombian Trial of Crenezumab Missed Primary Endpoints
- High-Dose Gantenerumab Lowers Plaque Load
- Amyloid Clearance: Check. Cognitive Benefit: Um … Maybe.
- Paper Alert: DIAN-TU Solanezumab and Gantenerumab Data Published
- Could Benefit of Plaque Removal Grow in Time?
Further Reading
News
- In DIAN-TU, Gantenerumab Brings Down Tau. By a Lot. Open Extension Planned
- Roche Pulls Plug on Two Phase 3 Trials of Crenezumab
- On Target: Crenezumab Reduces Aβ Oligomers in CSF
- Aducanumab, Solanezumab, Gantenerumab Data Lift Crenezumab, As Well
- A Close Look at Passive Immunotherapy Newbie, Crenezumab
- NIH Director Announces $100M Prevention Trial of Genentech Antibody
- Q&A With Ryan Watts, Genentech Lead Scientist on API Trial
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.