Donanemab Phase 3 Puts Plasma p-Tau, Remote Assessments to the Test
Quick Links
Donanemab, a monoclonal antibody trained against Aβ plaques, is hot on the heels of its slightly more advanced competitors aducanumab (see Part 1 of this series), and lecanemab (see Part 5) in the race for regulatory approval. Neck-in-neck with them: gantenerumab (see Part 6). So what's the latest? At this year’s Clinical Trials in Alzheimer’s Disease meeting, held November 9-12 in Boston and online, scientists from Eli Lilly and their academic partners parsed results from a completed Phase 2b study, reported baseline data from an ongoing Phase 3 trial, and showcased a new, decentralized approach of their prevention trial, also in Phase 3.
- TRAILBLAZER-2, a Phase 3 study, has enrolled 1,600 participants.
- Prescreening with plasma p-tau-181 helped select people with both amyloid and tau pathology.
- A secondary prevention trial, TRAILBLAZER-3, is designed to rely on remote assessments, not clinic visits.
Most of the discussion focused on how best to deploy tau measurements—both PET scans and blood tests—to pick out just the right participants for trials and maybe even gauge how well they will take to treatment. Scientists reported that plasma p-tau-181 works to select people likely to harbor both plaques and tau tangles in the brain, and that those who start on donanemab with few tangles benefitted the most from amyloid riddance. Donanemab slowed, but did not stop, tangle growth. In all, the findings underscored that treating earlier in the course of disease stands a better chance of curbing progression.
Donanemab targets a form of Aβ with a pyroglutamate modification on its N-terminus. Earlier data from the Phase 2 TRAILBLAZER study had shown that the antibody binds and clears its target, found only on plaques, remarkably well (see Mar 2021 conference news and Mintun et al., 2021). The trial enrolled 257 people who had an intermediate level of tau tangles as per tau PET, as well as amyloid accumulation above a threshold of presumed brain-wide abnormality set at 24 centiloids. Participants got monthly infusions of placebo or donanemab for 76 weeks, or until their amyloid levels dropped to 11 centiloids at one visit or below 24 for two consecutive scans. As reported before, 40 percent of treated people had “normal” levels of amyloid by 24 weeks, and 68 percent did by the trial’s end. Treatment slowed decline a tad, by 32 percent, on the integrated AD rating scale. The iADRS is Lilly’s customized composite of the ADAS-Cog and ADCS-iADL.
At AAIC, scientists reported that plasma p-tau-217 had edged down with treatment, and linked its reduction to slower cognitive decline. This cast this blood marker as an indicator of brain amyloid reduction and strengthened the tether between Aβ and tau in the amyloid cascade hypothesis of AD (Aug 2021 conference news).
Based on the data from its Phase 2 trial, Lilly announced late last month that it had started an FDA application for accelerated approval, which it will file by rolling submission of data as it comes in. It expects to complete this within the next few months. Lecanemab started the same process a month prior (Oct 2021 news).
At CTAD, scientists offered a deeper analysis of how amyloid and tau burden relate to treatment effect. Lilly’s Sergey Shcherbinin reported that, when used as a screening tool for the Phase 2 trial, tau PET selected not only participants with intermediate tau accumulation, but also with amyloid. After meeting the trial's cognitive criteria, people received a tau PET scan. Of those, 37 percent had an intermediate level of tangle accumulation and of those, 96.5 percent were subsequently found to have an amyloid plaque burden of at least 24 centiloids, meeting the trial's inclusion criteria.
Once enrolled, participants slid into the scanner for baseline scans, then again for more amyloid-PET scans every 24 weeks throughout the trial, and for a second tau PET scan at 76 weeks. Such serial data enabled Shcherbinin to see that the growth rate of tangle burden throughout the trial correlated with the degree of amyloid removal. Specifically, people whose amyloid burden had dropped below 24 centiloids by six months had less tau accumulation over the course of the trial than did people whose amyloid was only partially cleared by that time, although both groups benefited.
Breaking down the tau PET data regionally, Shcherbinin reported that donanemab treatment worked best at reducing tangles in various regions of the frontal lobe, which tends to get invaded after the temporal and parietal lobes have succumbed. The effect was striking in the frontal medial superior cortex, where donanemab curbed tau accumulation by nearly 100 percent in people who had cleared amyloid by 24 weeks, and by about 60 percent in people with partial early clearance.
Shcherbinin tied less tau progression in different lobes to the slowing of decline in different cognitive domains. For example, less tracer uptake in the temporal, parietal, and frontal lobes correlated with slightly better orientation, while tau reduction in the temporal and parietal lobes correlated with better word-finding. That said, none of these lobar tau measurements correlated with the global ADAS-Cog13 score.
Onward to Phase 3
Other scientists presented baseline data from the confusingly named TRAILBLAZER-2. This is a Phase 3 trial in people with early AD. Lilly's John Sims reported that, analogous to what had been done with plasma p-tau-217 in Phase 2, here the scientists adapted a plasma p-tau181 assay to the Simoa platform. Then they asked if it would work as a prescreening tool for enrollment in the Phase 3 trial.
Plasma p-tau-181 was measured among a subset of 752 potential enrollees before they had amyloid- and tau PET scans, while 3,619 other people had the PET scans without this prescreen. Among the 752, p-tau-181 predicted the presence of both amyloid and tau pathology. Sixty-three percent of those with elevated blood p-tau-181 turned out to have both plaques and tangles on PET, whereas only 37 percent harbored both types of deposit among those who got tau scans without prescreening. Sims said that in future trials, plasma pre-screening will help avoid unnecessary scans, reduce cost, and make trials accessible to people who live in regions without nearby PET centers.
Lilly’s Paul Solomon described the volunteers who are in this trial, which is fully enrolled. As of October 15, TRAILBLAZER-2 had randomized 1,625 participants. Nearly three-quarters live in the United States, the rest are in Japan, Canada, Poland, the Czech Republic, United Kingdom, The Netherlands, and Australia. It took screening more than 8,000 people to net these precious enrollees.
Solomon used this cohort's baseline data to compare demographic and disease features between people with intermediate versus high levels of tau pathology. Relative to people with intermediate tangle burden in either the Phase 2 or 3 studies, those with high tau in this new trial were younger. More of them were women, ApoE4 noncarriers, and on acetylcholinesterase inhibitors. They posted worse baseline scores on the MMSE, ADAS-Cog11, and CDR-SB, and were likelier to have mild AD than MCI. Interestingly, the baseline amyloid plaque burden between those with intermediate and high tangle burden was about the same. In all, this suggests that a person's tangle burden correlates with the stage of their clinical disease.
TRAILBLAZER-2 is designed much like the Phase 2 trial, which is typical for Phase 3 studies meant to confirm a Phase 2 efficacy signal. There is a notable difference, though, in that Lilly broadened the inclusion criteria to also include people with a lot of tau pathology, and indeed the baseline data appear to reflect a subcohort with somewhat “worse” AD.
Paul Aisen of the University of Southern California, San Diego, believes this might have been a mistake. He noted that even among people in the Phase 2 trial, which included only people whose tangle burden was intermediate, those on the higher end of this limited spectrum did not benefit from donanemab. “The data are pretty clear that the higher the baseline tau, the lower the response,” Aisen said. “It’s hard to understand the idea of broadening the inclusion criteria.”
Sims told Alzforum that including people with high tau, who tend to be younger and face a faster-progressing disease, will help future prescribers understand this population and whether they will benefit from donanemab. “Our goal for enrolling high-tau participants is to provide those answers while not jeopardizing the success of TRAILBLAZER-2,” Sims wrote. “We believe we can achieve both with our trial design, which will involve analyses that look at the intermediate tau group separately and also the total trial population.”
Separately at CTAD, several different neurologists besides Aisen questioned Lilly’s approach of stopping donanemab after amyloid scans normalize. They noted that while amyloid plaques remained at bay for some time after treatment discontinuation in Phase 2, there are indications that the disease could be picking up steam again. In the lecanemab Phase 2 trial, the blood Aβ42 to Aβ40 ratio—an indicator of amyloid accumulation—started to worsen again once participants were off that drug. “We may be relying too heavily on amyloid-PET, which is perhaps a slow responder to the effects of discontinuation,” Aisen said.
However, at CTAD, Sims made the case that plasma p-tau-217 stays low—for a year, at least—after donanemab treatment stops. Splitting the Phase 2 treatment groups into those who had cleared amyloid by 24 weeks and thus were moved to placebo at that time, and those who hadn’t, Sims reported that p-tau-217 stayed low until the end of the trial in both groups.
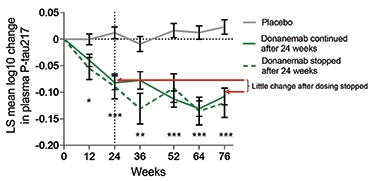
Go Down Stay Down? Plasma p-tau217 dropped in response to treatment with donanemab, and stayed low even among participants who stopped treatment at 24 weeks. [Courtesy of Eli Lilly & Co.]
Sims also noted that previous modeling studies suggest that following amyloid removal with donanemab, it would take three to four years for participants to return to amyloid-positive levels, and up to 14 years to return to their baseline plaque load. “It is important to test the hypothesis that once a pathology is removed that patients do not need to be subjected to continual therapy,” Sims wrote.
While TRAILBLAZER-2 expanded to fold in those with worse pathology, a third trial, TRAILBLAZER-3, is moving in the other direction. This secondary prevention trial—run with the Banner Alzheimer’s Institute in Phoenix—has started inviting people with amyloid accumulation but no clinical symptoms. At CTAD, Banner’s Pierre Tariot detailed its rationale and design.
Starting out with iADRS scores from donanemab's Phase 2 trial, split into tertiles of baseline tau PET, Tariot said those with the least tangles at baseline had benefited the most from treatment. Although everyone in this trial was already symptomatic, Tariot believes the data argue for treating people before they have tangles.
TRAILBLAZER-3 targets people in the preclinical stage, when amyloid is accumulating but symptoms have yet to surface. How to find such people is perhaps the field's most pressing question these days. For this trial, Lilly and Banner are using several tools to draw in potential participants. For one, they are tapping the Alzheimer’s Prevention Registry’s initiative, which has assembled 364,000 registrants. They are also bringing in recruits via clinicians across the United States who know interested patients, and through Lilly outreach efforts to community organizations, health centers, and pharmacies.
Candidates for the trial go through a screening process that does not require a single person to set foot inside a centralized study site, Tariot said. Rather, the trial embraced a new, decentralized design that relies on plasma biomarkers and remote assessments.
Recruits must be between 55 and 80 years old. They will be screened for lack of cognitive impairment via the modified telephone interview for cognitive status (TICS-m), a 13-item cognitive test that can detect amnestic mild cognitive impairment (Cook et al., 2009). These calls are made with centralized raters contracted by Lilly. Then, in a first for the field, plasma p-tau217 levels are used to screen for elevated brain amyloid in those who were deemed to be cognitively normal over the phone. Candidates can get their blood drawn for this test at designated sites near where they live, such as Quest Diagnostics. Those with elevated plasma p-tau217 complete the consent and enrollment process via video calls. Yes, that's all.
Once they are in, participants take their donanemab or placebo infusions at local, certified infusion centers. They will receive brain MRIs at baseline and two more times over the first five months of the trial, or as needed if concerning symptoms, such as those suggesting ARIA, emerge. Participants can get these scans at any certified MRI center. Tariot said there are few parts of the country without one, as MRI is more widely available than PET.

Blazing a Decentralized Trail? Most participants will never set foot inside a trial site. They receive infusions, blood draws, and MRIs at nearby locations, and take cognitive assessments via video call. A central study coordinator ushers them through the process, and a principal investigator oversees safety. [Courtesy of Eli Lilly.]
'Homework,' But No Appointments at the Clinic
Cognitive and neuropsychological assessments are going to be remote, Tariot said. Each participant will be assigned two central raters—one who will conduct the CDR-SB interview, and another who will conduct an array of other psychometric assessments, including the International Shopping List Test, Continuous Paired Associate Learning, Cognitive Function Index, and the Montreal Cognitive Assessment, which will serve as secondary outcomes. Both sessions happen every six months via video call. In addition to these raters, each participant gets a study coordinator who will usher them though the process of each assessment. Besides the rater-based assessments, participants will take several self-administered tests on study-issued tablets, which come with a hot spot to boost internet connectivity, if needed. Principal investigators are to oversee safety concerns of all participants within a given region.
The only time people may need to travel to a specialized center is for amyloid- or tau PET scans. Tariot said that the established sensitivity of plasma biomarkers has made this optional for participants. The trial aims to collect florbetapir-PET data on 200 people and flortaucipir-PET scans on 500 people. These participants are likelier to live in major cities where PET is available.
Hopefully, this approach will remove barriers to trial participation and speed up enrollment, Tariot said. Lilly and Banner are aiming for 3,300 participants. Enrollment is going smoothly, Tariot said, but he would not say how many people have joined, or how long he estimates filling the trial will take.
Hello Again, Time-to-Event?
While TRAILBLAZER-3 exemplifies a new design, its primary endpoint—time to emergence of cognitive impairment—harkens back to the early days of AD trials. Participants are randomized 1:1 to receive nine monthly infusions of placebo or donanemab. After that, infusions end and participants will be monitored every six months on their global CDR-SB, which tallies up impairment in six domains with memory considered the primary. This will continue until 434 participants have become cognitively impaired, i.e., scored above zero on the global CDR for two consecutive visits. Called 'time to progression to aMCI, this will be the trial's primary endpoint. Tariot said the groups' modeling predicts this will happen three years after enrollment is complete. The hope, clearly, is that those who progressed will have been on placebo.
After the trial's blinded portion ends, those who were on placebo can opt to receive nine doses of donanemab in the open-label extension. There are no plans to give another series of infusions to the original treatment group.
The trial's return to a time-to-event primary endpoint sparked debate. This used to be the norm in the field's early trials, but since has been discarded in favor of continuous measures. At CTAD, Michael Weiner of the University of California, San Francisco, questioned the choice. Previous studies had found that endpoints that incorporate changes in the rate of progression over time have more statistical power than those comparing singular events such as progression to MCI (Li et al., 2019). Aisen made a similar point, noting that the disease's gradual progression means discrete stages, and the binary decisions they require, are artificial. “When you separate disease progression into discrete stages to make a time-to-event design, you are throwing away the majority of the information as well as statistical power,” he told Alzforum.
Sims countered that Lilly is looking for a result that matters to people. “Nothing could be more meaningful than preventing progression to MCI,” he said. If disease is truly progressing over time in this preclinical population, then this endpoint should be able to detect a meaningful difference between treatment and placebo groups. Tariot told Alzforum that extensive mathematical modeling strongly suggested that the time-to-event endpoint was likeliest to detect a treatment effect in this study. This would imply that in those earlier time-to-event trials that were negative, the problems had been the drugs and insufficiently specific inclusion criteria, more than the endpoint.
But wait, there’s more. Lilly has plans for a third Phase 3 trial—TRAILBLAZER-4. It will compare the plaque-clearing prowess of donanemab to that of aducanumab head-to-head in 200 people with mild AD. That trial, which will use biomarkers of amyloid clearance as endpoints, is slated to start enrolling later this year.—Jessica Shugart
References
News Citations
- Aduhelm Lowers Tau; Registry to Track Real-World Performance
- Lecanemab Sweeps Up Toxic Aβ Protofibrils, Catches Eyes of Trialists
- Brain Shuttle Could Halve Amount of Gantenerumab Needed
- Donanemab Confirms: Clearing Plaques Slows Decline—By a Bit
- On Donanemab, Plaques Plummet. Off Donanemab, They Stay Away
- Lecanemab Follows Aduhelm’s Path to Accelerated Approval
Paper Citations
- Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704. Epub 2021 Mar 13 PubMed.
- Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009 Jun;22(2):103-9. PubMed.
- Li D, Iddi S, Aisen PS, Thompson WK, Donohue MC. The relative efficiency of time-to-progression and continuous measures of cognition in presymptomatic Alzheimer's disease. Alzheimers Dement (N Y). 2019;5:308-318. Epub 2019 Jul 18 PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Banner Alzheimer's Institute
In my view, this story does not represent the “Time to Event” debate entirely correctly. This is not a method used just in “early trials … but since has been discarded.” I and others, e.g. a lead statistician at Novartis, Angelika Caputo, have presented in a variety of settings that our extensive modeling for the Alzheimer’s Prevention Initiative Generation Program, also a prevention trial, showed that Time to Event actually had superior power to continuous measures. Our design paper summarized our approach (Lopez Lopez et al., 2019). Our modeling for Trailblazer 3, conducted independently, showed the same result.
References:
Lopez Lopez C, Tariot PN, Caputo A, Langbaum JB, Liu F, Riviere ME, Langlois C, Rouzade-Dominguez ML, Zalesak M, Hendrix S, Thomas RG, Viglietta V, Lenz R, Ryan JM, Graf A, Reiman EM. The Alzheimer's Prevention Initiative Generation Program: Study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer's disease. Alzheimers Dement (N Y). 2019;5:216-227. Epub 2019 Jun 12 PubMed.
Pentara Corp
In my experience, "Time to Event" looks as well-powered as a continuous endpoint only when you make the assumption that an X percent slowing in a continuous progression outcome is equivalent to the same X percent reduction in event rates. Although this may be true when you assume linearity of the decline over time, the true equivalence between disease slowing in an early stage of disease (with downward curvature) is closer to 15 percent event rate reduction, corresponding to 30 percent progression slowing, and 50 percent event rate reduction corresponding to 75 percent progression slowing (based on simulations that stretch out time to mimic a treatment effect).
So assuming that these values are the same inadvertently biases the power calculation in favor of “Time to Event” outcomes. Without this bias, “Time to Event” is only preferred when there is a bimodal distribution, which is very rarely the case. We recommend using a continuous progression outcome because it is nearly always more powerful than "Time to Event," especially in the early stages of disease when we observe downward curvature of continuous outcomes due to moving away from ceilings of scales.
Make a Comment
To make a comment you must login or register.