Chimeric Mice: Can They Model Human Microglial Responses?
Quick Links
Microglia are finicky. Take them out of their normal environment and they have a personality meltdown. They dramatically change gene expression and just about turn into a different type of cell. How, then, to study human microglia in a physiological setting? Enter the chimeric mouse. At this year’s AD/PD meeting March 27–31 in Lisbon, Portugal, Bart de Strooper, U.K. Dementia Research Institute at University College London, and Mathew Blurton-Jones, University of California Irvine, described how both their labs independently characterized human microglia grown inside the mouse brain. There, the cells thrive, apparently maintaining their human identity. They “tile” across the brain as normal, yet respond to stress differently than mouse microglia. “With this type of approach, we can begin to ask important questions about the function of human microglia and better understand how they interact with amyloid and tau pathology over time,” Blurton-Jones said.
- Researchers grow human microglia in mouse brains.
- The cells appear to retain their human expression profile.
- They seem to react to Aβ differently than do mouse microglia.
Scientists have long studied microglia in mice. Alas, while mouse and human microglia have similar transcriptional signatures initially, that changes with age (Jul 2017 news). Most recently, in a paper posted on bioRχiv on April 19, scientists led by Brad Friedman and David Hansen at Genentech, South San Francisco, report that human microglia from AD brains display a signature that differs considerably from disease-associated signatures seen in mice (Srinivasan et al., 2019).
In Lisbon, Renzo Mancuso from De Strooper’s lab at KU Leuven, Belgium, reported that, out of 39 AD risk genes identified in genome-wide association studies, eight, including the microglial receptors CR1 and CD33, have no clear mouse ortholog. Neither do 12 of 43 other genes linked to AD. For another 10 genes, including the microglial receptor and AD risk gene TREM2, the similarity between the mouse and human versions is low. Blurton-Jones agreed. “This is a major problem that makes it difficult to address important questions about the effects of AD risk genes on microglial function using traditional mouse models,” he said.
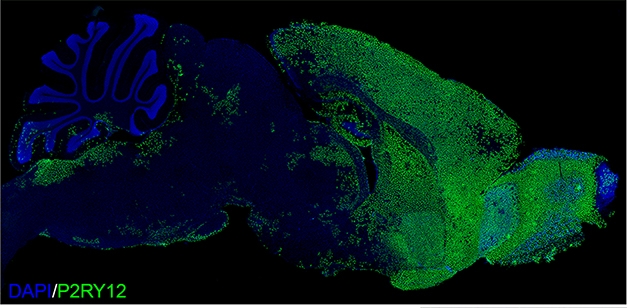
Chimeric Mouse. Two months after transplanting progenitors into mouse pups, human P2RY12-positive microglia had dispersed throughout the forebrain. [Courtesy of Morgan Coburn and Mathew Blurton Jones.]
Given these differences, researchers have turned to chimeric models. The strategy is to inject the brains of young mice with either human hematopoietic stem cells, or with microglia derived from human embryonic or induced pluripotent stem cells (Abud et al., 2017; Capotondo et al., 2017; Bennett et al., 2018). At AD/PD, De Strooper and Blurton-Jones characterized such transplants, including their transcriptional profiles and responses to Aβ and other forms of stress.
To deplete endogenous microglia, Mancuso treated pups with the colony stimulating factor 1 blocker BLZ945. CSF1 is an essential trophic factor for microglia, and BLZ945 halved their numbers. A day later, he injected human microglia derived from embryonic stem cells into the animals’ brains. The mice were Rag2- and IL2rγ-negative to preclude rejection of the human cells, and they expressed a humanized form of CSF1, since human microglia do not respond to mouse CSF1 (Rathinam et al., 2011). Eight weeks later, the human cells had taken on a seemingly normal, ramified appearance and distributed across the mouse brain in a classic microglial tiling pattern.
Even so, they kept their human transcriptome profile. Mancuso correlated expression profiles of thousands of human microglia isolated from mouse brain with profiles from thousands of cells isolated from temporal cortex tissue removed during neurosurgery. “The profiles overlapped completely,” said De Strooper.
Transcriptomes of human cortical microglia clustered into three main types designated homeostatic, cytokine responsive, and activated. The human cells grown in mice clustered in exactly the same way.
Jonathan Hasselmann and Morgan Coburn in Blurton-Jones’ lab took a slightly different approach. They also used immune-deficient, humanized CSF1 mouse pups, but instead of injecting microglia, they transplanted human iPSC-derived hematopoietic progenitor cells into their brain ventricles and overlying cortices. During the normal human development, these progenitors become microglia and other CNS myeloid cells within the brain, including perivascular, meningeal, and choroid plexus macrophages. Two months later, these researchers also saw the human microglia tile across the mouse forebrain, while a smaller number of human brain macrophages lined mouse blood vessels, meninges, and the choroid plexus. About three-quarters of the microglia were human. Their transcriptional signatures mirrored those of microglia freshly isolated from human brain (Jun 2017 news on Gosselin et al., 2017).
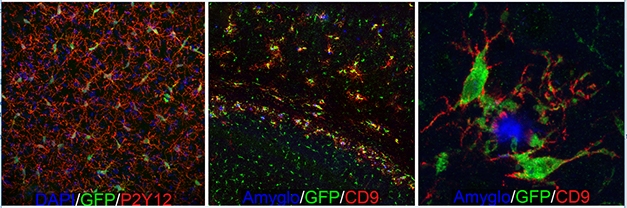
Microglial Dynamics. GFP-expressing human microglia tile across the forebrain (left) and extend highly ramified processes (red) indicative of a homeostatic state. When transplanted into immune-deficient 5xFAD mice (middle, right), the human microglia migrate towards Aβ plaques (blue) and upregulate expression of the DAM marker CD9 (red) [Courtesy of Morgan Coburn and Mathew Blurton-Jones.]
How would the cells respond to Aβ? Mancuso injected 5 μL of 10 μM synthetic oligomers into the mouse brain ventricles eight to 10 weeks after transplanting the human microglia. In reaction to this insult, endogenous mouse microglia shifted their transcriptomes sequentially from homeostatic to cytokine responsive, to activated, with the latter partially overlapping with disease-associated microglial (DAM) signatures described previously (Jun 2017 news). Human microglia in the mice underwent a transcriptional transformation as well, but analysis of more than 10,000 orthologous genes indicated poor correlation between human and mouse microglial responses. Of 207 differentially activated genes, 112 were up in human but not mouse microglia; they included GWAS hits BIN1 and PICALM. “We saw that human microglia responded very differently to Aβ oligomers. This emphasizes the need to look specifically at human cells in the context of AD,” said De Strooper.
Blurton-Jones and colleagues took a different approach by breeding their immune-deficient hosts with 5xFAD mice, then transplanted the human cells into the crosses and tested how the human microglia behaved. In mice that accumulated amyloid plaques, the response was robust and highly localized (see image above). Nine months after injecting human cells, microglia that were right next to a plaque became more amoeboid, downregulated homeostatic genes, and upregulated several DAM genes, as determined by RNA-Seq, including HLA-DRB1, CD9, TREM2, and CD11c. Blurton-Jones said that the human chimeric cell signature only partially overlapped with the mouse DAM signature. Of the 221 differentially expressed genes in plaque-associated human microglia, only about 10 percent match those that are up- or downregulated in the mouse DAM signature. However, Blurton-Jones thinks additional work needs to be done to validate these new genes in human tissue. Some differences could come down to methodology.
Still, De Strooper and Blurton-Jones believe that these chimeric models for now are the way to go to study human microglia in model settings. Of the 30 AD-linked genes with no or poor mouse orthologs, Mancuso found 25 expressed in microglia extracted from patients and 23 expressed in human microglia transplanted into mice. “This emphasizes the importance of using human-specific systems to interrogate genotype-phenotype interactions of the GWAS-identified AD risk genes,” De Strooper noted.—Tom Fagan
References
News Citations
- Human and Mouse Microglia Look Alike, but Age Differently
- What Makes a Microglia? Tales from the Transcriptome
- Hot DAM: Specific Microglia Engulf Plaques
Paper Citations
- Srinivasan K, Friedman BA, Etxeberria A, Huntley MA, van der Brug MP, Foreman O, Paw JS, Modrusan Z, Beach TG, Serrano GE, Hansen DV. Alzheimer's Patient Microglia Exhibit Enhanced Aging and Unique Transcriptional Activation. Cell Rep. 2020 Jun 30;31(13):107843. PubMed.
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017 Apr 19;94(2):278-293.e9. PubMed.
- Capotondo A, Milazzo R, Garcia-Manteiga JM, Cavalca E, Montepeloso A, Garrison BS, Peviani M, Rossi DJ, Biffi A. Intracerebroventricular delivery of hematopoietic progenitors results in rapid and robust engraftment of microglia-like cells. Sci Adv. 2017 Dec;3(12):e1701211. Epub 2017 Dec 6 PubMed.
- Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, Plowey ED, Barres BA. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron. 2018 Jun 27;98(6):1170-1183.e8. Epub 2018 May 31 PubMed.
- Rathinam C, Poueymirou WT, Rojas J, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rongvaux A, Eynon EE, Manz MG, Flavell RA. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011 Sep 15;118(11):3119-28. Epub 2011 Jul 25 PubMed.
- Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JC, Sajti E, Jaeger BN, O'Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017 Jun 23;356(6344) Epub 2017 May 25 PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.