Staging of Alzheimer’s, the Second: Neurodegeneration Does Not Equal Tauopathy
Quick Links
The use of biomarkers has transformed scientists’ view of Alzheimer’s disease, revealing that pathology begins to accumulate more than 20 years before clinical symptoms. This knowledge paved the way for secondary prevention trials. Now, tau PET is further changing conceptions of the disease. The new data also challenge the view that tau accumulation and neurodegeneration are synonymous. In fact, in many older people, the brain atrophies in the absence of neurofibrillary tangles. At the Alzheimer’s Association International Conference 2016, held July 22-28 in Toronto, Cliff Jack of the Mayo Clinic, Rochester, Minnesota, argued that AD staging criteria need to change to account for this new knowledge.
Jack proposed a new classification system that considers tau pathology separately from markers of neurodegeneration, such as brain atrophy and hypometabolism. In this scheme, all older adults could be classified based on the presence or absence of three key markers: amyloid-β (A), neurofibrillary tangles (T), and neurodegeneration (N). This ATN system would be a descriptive classification scheme rather than a diagnostic one, Jack said. It does not include cognitive status, although he suggested that could be added later as a fourth variable. Such a system might better describe people with suspected non-AD pathology (SNAP), who fall out of the current classification method, he noted. Jack described the proposed system in the Aug 2 Neurology, in a paper co-authored by many other experts in the imaging and biomarker fields.
Overall, researchers at AAIC expressed enthusiasm for the proposal and said they think the field needs a system like this. Some questioned whether continuous variables can truly be dichotomized as either present or absent. Several talks at AAIC supported this idea of a cutoff to define negative and positive imaging scans. Others stressed the importance of using multiple biomarkers to pin down a diagnosis. Victor Villemagne of the University of Melbourne, Australia, appreciated the ATN scheme, telling Alzforum, “Combining biomarkers will increase [diagnostic] specificity.”
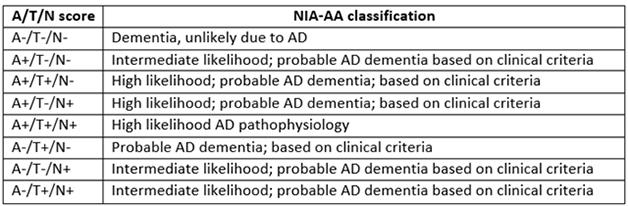
Refining AD Classification. The proposed ATN system divides people with dementia according to their biomarker profile, allowing for more nuance than the NIA/AA system.
Evolving Disease Concepts
The current staging criteria for Alzheimer’s have been around for only about five years. In 2011, the National Institute on Aging and the Alzheimer’s Association debuted new diagnostic guidelines for AD that recognized preclinical and prodromal phases and used biomarkers to stage patients for research purposes (see Aug 2010 conference news; Apr 2011 news). In the preclinical period, stage 1 was marked by amyloid accumulation alone, stage 2 by amyloid plus a marker of neurodegeneration or neuronal injury, including tau accumulation, and stage 3 by those markers plus subtle cognitive impairments. Meanwhile, a separate effort by the International Working Group for New Research Criteria for the Diagnosis of Alzheimer’s Disease, spearheaded by Bruno Dubois at Pierre and Marie Curie University, Paris, defined a distinct but similar system (see Jun 2011 webinar). In several longitudinal studies, the NIA/AA criteria proved useful for predicting who among a cognitively healthy group was likely to progress to cognitive impairment and dementia (see Aug 2013 conference news; Dec 2014 conference news; Apr 2016 news).
However, real-world data also made clear that many people did not fit into this classification scheme, because they had markers of neurodegeneration in the absence of amyloid. Called SNAP, this group had a distinct prognosis. Most people with SNAP maintain relatively stable cognition over time, progressing slowly if at all, although some studies have reported exceptions (see Aug 2013 conference news; Sep 2015 conference news). Now, tau imaging has found that most people in this group lack neurofibrillary tangles, as well as amyloid, Jack noted. Instead, they seem to suffer from a diverse array of neurodegenerative conditions or brain injuries.
The ATN system would better reflect this reality, Jack suggested. In this scheme, the presence of amyloid would be determined by amyloid PET or analysis of CSF Aβ42, and neurofibrillary tangles by tau PET or CSF phospho-tau. More generic neurodegeneration would be marked by hippocampal atrophy, hypometabolism in the brain, or CSF total tau. Some scientists have objected to the separation of CSF ptau and t-tau, Jack noted. However, studies by Kaj Blennow and colleagues at the University of Gothenburg, Sweden, as well as others, indicate that total tau reflects neuronal injury, as it spikes after head trauma or stroke. Phosphorylated tau, on the other hand, signals the presence of neurofibrillary tangles, and is more specific for AD than total tau or even CSFAβ42, Henrik Zetterberg at Gothenburg noted in a talk at a separate session. CSF Aβ42 levels drop in neuroinflammatory conditions and normal-pressure hydrocephalus, while ptau does not rise in non-AD tauopathies, Zetterberg said.
Most researchers who have worked on Alzheimer’s classification schemes now support separating tau pathology from neurodegeneration, Jack claimed. Evidence for this separation has been building for a while (e.g., Sep 2015 conference news on total tau). However, some point out that markers of degeneration, such as structural MRI and FDG PET, can disagree with each other and may be measuring different things. Jack argued that these markers reflect a similar underlying process, which is the loss of synapses and neurons. Other researchers at AAIC suggested that “neurodegeneration” is a misnomer, and the third category in the ATN system should perhaps be called brain injury or damage instead, reflecting the fact that it typically does not progress.
Jack did not address how the new ATN system affects the models of AD biomarker progression that have become widely used in the field (see Jan 2010 webinar). There is also no data yet on how existing cohorts such as ADNI would break out under this categorization system. Jack suggested, however, that because the ATN model does not assume anything about the order in which biomarkers appear, or what pathology causes which symptoms, it could better capture the varied diseases that can afflict the brain. “More goes wrong with the aging brain than just Alzheimer’s disease,” he noted.
In an August 2 Neurology editorial on Jack’s paper, Alison Murray of the University of Aberdeen, U.K., praised the system’s reliance on objective biomarkers rather than clinical judgments of cognitive impairment for staging AD. “[This] can only be helpful,” she wrote, noting that factors such as education and early life experience can confound staging by cognitive assessments. She suggested that future iterations of the system should include biomarkers for vascular disease and Lewy body accumulation as well, to more fully encompass all pathologies of aging.
Making Sense of SNAP
One of the main inspirations for the new system was to decipher the puzzle posed by the SNAP group, Jack told Alzforum. The ATN system divides the heterogeneous SNAP group into three categories, according to whether a person has neurodegeneration only, tau pathology only, or both. This may help researchers pin down underlying conditions. Because the ATN system is untested, Jack had no data on what proportion of the SNAP group might fit into each category. He suggested that some people in this group could have primary age-related tauopathy (PART) and be “T” positive. Because PART accumulates the same type of tau aggregate that AD does, the deposits would be detectable by tau scans. Others might be “N” positive due to any of several conditions such as vascular disease, TDP-43 or Lewy body accumulation, hippocampal sclerosis, or argyrophillic grain disease, Jack said. Some of these diseases would be expected to progress quite slowly, as SNAP does.
The question of what makes up SNAP matters because the group is large. In the Mayo Clinic Study of Aging, which comprises roughly 450 participants, one-quarter are classified as SNAP, Jack said at AAIC. Other studies report similar numbers. A recent paper from the Australian Imaging, Biomarker, and Lifestyle (AIBL) study bolsters the view of SNAP as a collection of slowly progressing disorders. Almost a quarter of the 573 cognitively healthy AIBL participants are classified as SNAP based on low hippocampal volume and absence of amyloid. Although this group had slightly lower cognitive scores at baseline compared with people without any pathology, they did not decline any more than controls over the six years of the study. Only 9 percent of them developed amnestic mild cognitive impairment or AD, about half the progression rate of the amyloid-positive groups (see Burnham et al., 2016).

A MeTeR for Tau. Tau tracer binding in three composite regions may determine whether a person is positive or negative for tangles. [Courtesy of Victor Villemagne.]
Can Yes/No Calls Capture Complex, Mixed Disease?
The objections to defining biomarkers as positive or negative center around the idea that any threshold is arbitrary, hence questionable. Jack counters that medicine uses arbitrary thresholds for many other continuous biomarkers—blood pressure, cholesterol, blood glucose. William Jagust at the University of California, Berkeley, agreed. “A lot of researchers don’t like the idea of classifying people as positive and negative on all these markers. But I think we have to do it,” he told Alzforum.
Other talks discussed specific methods for setting thresholds for positivity. Shruti Mishra of Washington University School of Medicine, St. Louis, described a data-driven method to define a pattern of tauopathy that distinguishes disease stages. She took AV1451 scans from 84 cognitively normal participants in WashU aging studies and applied a clustering algorithm to find the brain regions that best separated people into high and low tau signal groups. Four regions stood out: the entorhinal cortex, lateral occipital cortex, inferior temporal lobe cortex, and precuneus. From these she calculated a summary measure. Higher values correlated with lower scores on tests of episodic, visuospatial, and attentional memory. In these regions, an AV-1451 SUVR cutoff of 1.28 cleanly separated amyloid-positive and -negative participants, Mishra reported. Recent studies report that tau becomes widespread only in the presence of plaques, so the two pathologies often go hand-in-hand (see Jul 2016 news).
In the AIBL study, researchers use a similar method for determining the tau imaging cutoff. Chris Rowe of the University of Melbourne explained the “MeTeR” scale, based on tau pathology in three defined regions: a mesial temporal composite (M), a temporoparietal composite (T), and the rest of neocortex composite (R). Tau levels in two of these three regions, as seen with either AV1451, THK5317, or THK5351, have to be in the top 25 percentile of the cognitively normal group to be considered “positive.” This measure distinguishes cognitively normal participants from cognitively impaired and AD patients, Rowe reported. According to the MeTeR scale, high regional and global tau levels associated with worse performance on all cognitive tests. In addition, everyone who had high cortical tau (the T and R regions) also had high amyloid, as in Mishra’s study. In the SNAP group, the percentage of tau-positive people depended on their clinical classification. About 10 percent of those who were cognitively healthy had high tau, but among those with mild cognitive impairment this rose to 40 percent.—Madolyn Bowman Rogers
References
News Citations
- Revised Criteria for Preclinical AD, Exactly as Presented
- Revised Diagnostic Criteria for Alzheimer’s Are Published
- Biomarkers Predict Alzheimer’s, But Shoe Does Not Always Fit
- Large Studies Agree: Brain Amyloid Accelerates Cognitive Decline
- CSF Tau Rivals Aβ for Predicting Cognitive Decline
- Suspected Non-Amyloid Pathology (SNAP)—Not an Open or Shut Case
- Suspected Non-Alzheimer Pathophysiology: It’s Not Exactly a Snap
- Do Temporal Lobe Tangles and Cortical Plaques Together Bring on Alzheimer’s?
Webinar Citations
- Two New Sets of Diagnostic Criteria—Which Is Right for Your Clinic?
- Together at Last, Top Five Biomarkers Model Stages of AD
Paper Citations
- Burnham SC, Bourgeat P, Doré V, Savage G, Brown B, Laws S, Maruff P, Salvado O, Ames D, Martins RN, Masters CL, Rowe CC, Villemagne VL, AIBL Research Group. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: a longitudinal study. Lancet Neurol. 2016 Sep;15(10):1044-53. Epub 2016 Jul 20 PubMed.
Further Reading
News
- On Multiple Marker Analysis, Tangles Track Best With Functional Decline
- On Multiple Marker Analysis, Tangles Track Best With Functional Decline
- Countdown to Alzheimer’s: Can Fluid Biomarkers Predict Progression?
- Tau PET Fits With CSF, Grows Over Time, Picks up Frontotemporal Cases
- Tau PET Aligns Spread of Pathology with Alzheimer’s Staging
- Tau Tracers Track First Emergence of Tangles in Familial Alzheimer’s
- Next Up for Human Brain Imaging: Synaptic Density?
Primary Papers
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016 Aug 2;87(5):539-47. Epub 2016 Jul 1 PubMed.
- Murray AD. A new biomarker classification system for AD, independent of cognition: Agnosticism is a start. Neurology. 2016 Aug 2;87(5):456-7. Epub 2016 Jul 1 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Neuronetrix
In Jack et al., the authors propose a broad new biomarker-based disease classification scheme that could be useful in AD and other dementing disorders. This is an important paper, where the authors encourage the whole community to step back and re-examine the scientific framework for assessing affected patients and describing the disease-related effects.
While the current IWG and NIA-AA biomarker classification systems are linked to a specific disease hypotheses, and are based on consensus diagnostic criteria, the authors propose a new descriptive system for categorizing multi-domain biomarker findings at the individual person level. In their proposed A/T/N classification system, the AD biomarkers are divided into three binary classes for Aβ and tau pathology and neurodegeneration biomarkers. This is a forward-thinking approach that relies on objective measures, is easy to understand and use, is applicable across all clinical diagnostic states, and in the long run will provide a more complete clinical picture of dementing disorders.
As suggested in the manuscript, the classification system would benefit from the inclusion of a separate category for synaptic dysfunction (S). Synaptic loss leads to a breakdown in the brain’s bioelectric neural network that is central to neurodegenerative disorders (Herrup, 2015; Fox, 1999).
While the authors suggest the use of several functional, biochemical, and anatomical biomarkers of synaptic dysfunction, it may be useful to broaden the focus and include measures of the bioelectrical activity of large-scale synaptic networks (E) such as event-related potentials (ERPs) and quantitative EEGs (QEEGs). These tests reflect the information-processing functions of the brain and are not captured using the other proposed modalities. While these electrophysiologic biomarkers historically have been difficult to capture in a practical and cost-effective way, technological advances now allow for reliable, easy-to-perform, and inexpensive ERP and QEEG testing (Cecchi et al., 2015) that can provide Information complementary to A/T/N biomarkers, leading in the end to a better, more complete classification scheme; A/T/N/S/E.
References:
Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015 Jun;18(6):794-9. PubMed.
Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999 May 12;52(8):1687-9. PubMed.
Cecchi M, Moore DK, Sadowsky CH, Solomon PR, Doraiswamy PM, Smith CD, Jicha GA, Budson AE, Arnold SE, Fadem KC. A clinical trial to validate event-related potential markers of Alzheimer's disease in outpatient settings. Alzheimers Dement (Amst). 2015 Dec;1(4):387-94. Epub 2015 Oct 2 PubMed.
Make a Comment
To make a comment you must login or register.