On Donanemab, Plaques Plummet. Off Donanemab, They Stay Away
Quick Links
The FDA’s controversial approval of aducanumab hinged on the premise that clearance of amyloid would be “reasonably likely” to bestow a cognitive benefit. Data presented at the Alzheimer’s Association International Conference (AAIC), held July 26-30 in Denver and online, support the idea that two other antibodies could clear that low bar, as well. Scientists from Eli Lilly reported that the plaque-dissolving strength of donanemab, an antibody trained against forms of Aβ detectable only in plaques, tracked closely with plummeting plasma p-tau217. Weaving their data into a disease-progression model that had been generated from past trial data, they claimed that the amyloid- and tau-lowering effects of the drug correlated with a slowing of cognitive decline. Separately, data from lecanemab’s Phase 2 trial and open-label extension studies provided yet more support for that antibody’s disease-modifying effect, despite the travails that have beset its path through clinical development.
- In a Phase 2 trial of donanemab, Aβ plaques and plasma p-tau217 fell in unison.
- These shifts correlated with slower cognitive decline decline based on a disease progression model generated with historical controls.
- After amyloid clearance and switch to placebo, plaque levels remained low for at least a year.
“I was mightily encouraged by the data presented by Lilly and Eisai, which gave further support for the FDA assertion that there was a ‘reasonable likelihood’ that lowering the Aβ-load would result in clinical benefit,” commented Colin Masters of the University of Melbourne, Australia.
Both donanemab and lecanemab have received breakthrough therapy status from the FDA. Similarly to aducanumab, this means that their sponsors could apply for accelerated approval based primarily on changes in surrogate biomarkers that demonstrate amyloid reduction. Both Lilly and Biogen/Eisai have announced plans to use their Phase 2 data to apply for accelerated approval of donanemab and lecanemab, respectively. Lilly will file later this year (see Endpoints News).
“Lilly is in the catbird seat with donanemab,” said Lon Schneider of the University of Southern California in Los Angeles. He noted that Lilly’s Phase 2 trial met its primary endpoint, and its biomarker data showing both amyloid and tau reduction were more convincing than Biogen’s on aducanumab. Even so, Schneider continues to disagree with the new precedent set by the FDA, and favors that Phase 3 clinical efficacy be demonstrated prior to approval of any therapeutic antibody.
Alzforum covered results of the Phase 2 donanemab trial—called TRAILBLAZER—in March, just as the data was also published (Mar 2021 conference news; Mintun et al., 2021). At AAIC, John Sims and Mark Mintun of Lilly reported fresh analyses since then of the amyloid and tau biomarker data, respectively.
The trial enrolled 257 participants who had early symptomatic AD, amyloid in their brains, and—notably—an intermediate level of neurofibrillary tangles based on PET scan. After an initial period, when 131 volunteers randomized to the treatment group gradually received higher and higher doses of donanemab, the trial settled in on monthly infusions of 1,400 mg donanemab. This was given until a person’s amyloid burden dropped below 25 centiloids—the level in healthy young controls—at which point the dose was lowered to 700 mg. If amyloid fell below 11 centiloids, or below 25 for two consecutive scans, the person was switched to placebo.
As previously reported, the 76-week trial met its primary cognitive endpoint, showing a 32 percent slowing of decline on the Integrated Alzheimer’s Disease Rating Scale (iADRS). By 24 weeks, donanemab had completely cleared plaques in 40 percent of participants in the treatment group; by the trial’s end, 68 percent had reached normal levels.
At AAIC, Sims broke down that amyloid reduction data further. Most participants in the treatment group had a rapid reduction in amyloid over the first 24 weeks. As might be expected, the amount of amyloid they shed over those first six months depended upon how much they started with, Sims reported. In other words, people with higher levels of amyloid at baseline lost more than those who started with less. Still, they took longer to dip into the realm of healthy young controls.
Sims reported that once a person’s amyloid complete cleared, their levels stayed down for the remainder of the trial. Among the participants with “deep amyloid clearance,” i.e., amyloid levels below 11 centiloids, and who were switched to placebo by 24 weeks, amyloid burden crept up only slowly by 76 weeks, barely cresting 11 centiloids, on average. At this rate, it would take 14 years for amyloid to accumulate back to baseline level for this group, or about 90 centiloids, Sims reported (see image below).
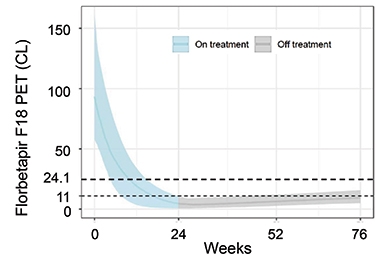
Get Out, Stay Out. Among people who had complete removal of amyloid plaques by 24 weeks, and were switched to placebo at that time, amyloid burden stayed within the range of healthy young controls (less than 25 centiloids) for the remainder of the trial. [Courtesy of Eli Lilly.]
How did this rapid amyloid clearance influence downstream parts of the AD cascade, i.e., tau tangles and cognitive decline? All participants underwent flortaucipir-PET scans at baseline and 76 weeks. Sims reported that compared to the placebo group, those who had had complete clearance of amyloid by 24 weeks had a significant dip in tangle burden in the temporal, parietal, and frontal lobes by the end of the trial. People who had had partial amyloid clearance by 24 weeks also had significant drops in tau tracer uptake, at least in the parietal and frontal lobes. “These findings link us back to the concept of AD as an amyloid-induced tauopathy,” Sims said.
Would riddance of amyloid also track with slowing of cognitive decline? Among all participants, including those in the placebo group, the percent change in amyloid burden by 24 weeks had no significant correlation with the change in iADRS scores between baseline and 52, 64, and 76 weeks, though there was a trend toward slower cognitive decline among those who had cleared the most amyloid.
Given the small numbers of participants in the Phase 2 trial, Sims further analyzed the trial data using a disease-progression model developed at Pfizer that was based on data from nearly 5,000 controls (Conrado et al., 2014). The Coalition Against Major Diseases had gathered this control data, including from the placebo arms of 15 AD clinical trials conducted between the 1990s and 2010 on people who had mild to moderate AD (Jul 2013 news; Dec 2010 news). The model calculates trajectories of disease progression—as gauged by ADAS-Cog scores—among populations with different characteristics, such as ApoE4 carriers or noncarriers.
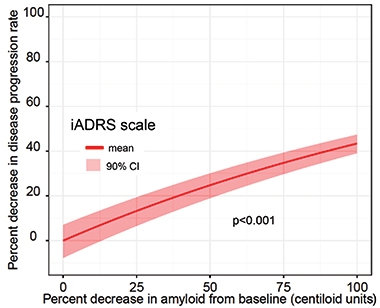
Model Comparison. By plugging percent amyloid reduction into the Conrado model, Sims found a statistically significant relationship between amyloid clearance and slowing of disease progression. [Courtesy of Eli Lilly.]
When Sims plugged data from TRAILBLAZER into the model, it estimated that donanemab slowed cognitive decline by 28 percent among all participants in the treatment group, and by 42 percent among APOE4 carriers. The findings jibe with the 32 percent slowing of cognitive decline that the researchers had previously reported, though the model lowered the p value, at less than 0.001. The model also rendered a statistically significant link between amyloid reduction and disease slowing.
Schneider pointed out that the model used data from people who had mild to moderate AD, whereas TRAILBLAZER selected participants with MCI to mild AD. “So there’s a good chance that many of the inputs from the TRAILBLAZER trial are where the model is least accurate,” Schneider said. Still, he and other researchers felt that the Lilly researchers were doing their best with the limited data available.
Plasma p-tau217 Tracks with Amyloid Drop
Mintun’s presentation focused squarely on freshly garnered data from Lilly’s in-house plasma p-tau217 assay. Lilly originally developed the assay on the Meso Scale Discovery platform, which works like an ELISA. At AAIC, Mintun debuted the assay’s adaptation to the more sensitive Simoa platform. Several groups have reported that p-tau217 in the CSF and plasma rise early in the disease trajectory and correlate with amyloid and tangle load (Apr 2020 conference news; Jul 2020 conference news). In a first for the field, Mintun reported how plasma p-tau217 levels changed in response to treatment.
At baseline, plasma p-tau217 correlated with baseline amyloid-PET and tau-PET measures as reported previously. This may seem like a no-brainer, but the result is striking considering that trial enrollment was restricted to people with an intermediate level of tau tracer uptake, thus limiting the dynamic range of PET measurements at baseline, Mintun said.
What happened to p-tau217 as brain amyloid plummeted in response to donanemab treatment? In short, it followed suit. Mintun reported that by 12 weeks, plasma p-tau217 was already significantly lower in the donanemab group compared to placebo. Levels continued to fall at every time point, while statistical significance strengthened. By the end of the trial, plasma p-tau217 had fallen by 24 percent in the treatment group, and risen by 6 percent in the placebo group. The latter rise is on par with that expected during disease progression, Mintun said.

Plasma p-Tau217 Plummets. In response to donanemab treatment, amyloid plaque burden (left) and plasma p-tau217 dropped throughout the trial. [Courtesy of Eli Lilly.]
Notably, at 24, 52, and 76 weeks, the drop in plasma p-tau217 from baseline was strongly tied to reduction in amyloid. In addition, reduction in p-tau217 was linked to reduction in tau tangles, according to tau-PET, between the baseline and the end of the trial.
Mintun also applied the p-tau217 data to the disease progression model that Sims had employed. This cast change in plasma p-tau217 as a significant predictor of slowed disease progression.
Henrik Zetterberg of the University of Gothenburg in Sweden called the responsiveness of plasma p-tau217 to donanemab treatment “a Hallelujah moment,” noting that this was the first time rigorous plasma p-tau biomarker data had been presented in the context of a clinical trial. He said the findings provide strong support for the amyloid cascade hypothesis. That p-tau217 dropped so quickly in response to amyloid removal supports the idea that neurons actively release phospho-tau in response to exposure to amyloid. “If we reduce amyloid exposure, we would expect to see a very quick decrease in phospho-tau secretion,” Zetterberg said. “And that’s exactly what we see.”
This data was also a meeting highlight for Oskar Hansson of Lund University, Sweden. “These important results clearly indicate that p-tau217 in plasma might be used as an easily accessible and cost-effective marker revealing effects of novel treatments on the levels of amyloid-β fibrils in the brain,” Hansson wrote. However, he emphasized that it is not yet known whether plasma p-tau217 can be used as a surrogate biomarker of clinical efficacy. “We simply do not yet know if the reduction of extracellular levels of soluble p-tau is an epiphenomenon merely associated with a reduction in β-amyloid fibrils in the brain, or a key event that will always lead to diminished tau aggregation, less neuronal dysfunction, and reduced degeneration, independently of the mechanism causing the reduced levels of extracellular p-tau to begin with.”
Paul Aisen of the University of Southern California in San Diego made a similar point. “The treatment-related fall in p-tau217 must still be linked to clinical benefit as validation of its utility in guiding therapy,” he wrote. “But if it can be validated, this assay will be an enormously useful tool in the drug-development process.”
David Morgan of Michigan State University in Grand Rapids, who attended the meeting in person, asked Mintun if and when they would measure other blood biomarkers that could hint at a neuroprotective effect of treatment, such as neurofilament light (NfL) and glial fibrillary acidic protein (GFAP). Absent solid cognitive data, perhaps these markers could get closer to addressing the question of efficacy, Morgan told Alzforum. Mintun said that they intend to measure other markers, but optimizing the p-tau217 assay on the sensitive Simoa platform had been top priority leading up to the meeting.
Zetterberg believes this effort may have been well worth it. Given the vanishingly small concentration of p-tau217 in the plasma, the added boost of sensitivity may have been crucial for detecting the treatment effect. Zetterberg said that while he looks forward to seeing how neurodegenerative biomarkers respond to donanemab treatment, the data could prove difficult to interpret. “As microglia are recruited to donanemab-bound plaques, GFAP levels might go wild,” he said. NfL could even rise for a time, he added, as glial cells nosh on detritus from amyloid-exposed neurons. Zetterberg acknowledged that while potentially interesting and important, complex biomarker data might be the last thing Lilly wants while seeking accelerated approval of donanemab by the FDA.
The most exciting implication of the donanemab data, Zetterberg said, was the possibility of treating patients for only a few months to remove plaques. Then, plasma p-tau markers could be used to monitor patients for the return of plaques, at which point they could take another round of treatment, he suggested. Zetterberg was heartened by the data showing that neither Aβ plaques nor plasma p-tau217 appeared to rise within a year after donanemab treatment stopped.
If approved, “amyloid plaque removal” could become a common treatment for people in their golden years who go to their doctors with cognitive complaints, Schneider said.
What about for cognitively normal people who have amyloid plaques? Could donanemab be approved for them? For now, aducanumab is only conditionally approved for people in the early symptomatic stages of AD. Both donanemab and lecanemab trials have included only this population as well. That will soon change, however, when TRAILBLAZER-ALZ3 begins. This Phase 3 trial will test donanemab in cognitively unimpaired people at risk for AD, and will use plasma p-tau217 to select participants (Jul 2021 news).
Lecanemab is also being put to the test in people with preclinical AD in the AHEAD3-45 trial, which is enrolling participants. At AAIC, Chad Swanson of Eisai presented data from the Phase 2 study of lecanemab and an 18-month open label extension.—Jessica Shugart
References
News Citations
- Donanemab Confirms: Clearing Plaques Slows Decline—By a Bit
- AD Trial Simulation Tool Receives Regulators’ Blessings
- DC: CAMD Convenes Stakeholders to Reform Alzheimer’s Trials
- 217—The Best Phospho-Tau Marker for Alzheimer’s?
- Plasma p-Tau217 Set to Transform Alzheimer’s Diagnostics
- Can Donanemab Prevent AD? Phase 3 TRAILBLAZER-ALZ3 Aims to Find Out
Paper Citations
- Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704. Epub 2021 Mar 13 PubMed.
- Conrado DJ, Denney WS, Chen D, Ito K. An updated Alzheimer's disease progression model: incorporating non-linearity, beta regression, and a third-level random effect in NONMEM. J Pharmacokinet Pharmacodyn. 2014 Dec;41(6):581-98. Epub 2014 Aug 29 PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
University of Melbourne
I was mightily encouraged by the data presented by Lilly and Eisai, which gave further support for the FDA assertion that there was a “reasonable likelihood” that lowering the Aβ-load would result in clinical benefit.
For those with “normalization” of Aβ-PET by donanemab at 24 weeks, tau-PET reduction was more effective at 76 weeks, and there was less clinical decline. Using updated exposure/response and progression models, those whose Aβ-PET levels fell below 11CL, treatment could cease, and it could be predicted that it would take 14 years or longer for it to return to abnormal levels.
Moreover, there was a direct relationship between lowering Aβ load and slowing disease progression (28 percent slowing overall, 42 percent slowing in APOE4 carriers). Aβ load lowering predicted disease slowing.…More
These relationships held for an in-house p-tau217 immunoassay (capture p-tau217 IBA493, detection tau 4G10E2), which showed excellent sensitivity to therapeutic intervention with donanemab, as early as 12 weeks after administration. Plasma p-tau217 decreased by a notable 29 percent at 76 weeks, the largest effect seen in those with Aβ-PET levels reduced to baseline or below (meaning that the baseline at 24CL had been set too high, and that 11CL is more realistic).
Eisai’s lecanemab demonstrated the utility of the C2N “Procivity” IP-MS assay for the normalization of the plasma Aβ42/40 ratio in both the double blind phase and open label extension (OLE) studies. This answers a question that has been hanging over the field for the last decade: Will administration of monoclonal antibodies to Aβ destroy the driving changes in plasma of lower Aβ42 levels in the natural history of AD? Apparently not, at least as far as lecanemab is concerned, where the plasma Aβ42/40 ratios were shown to be a sensitive marker of efficacy, particularly after cessation of therapy and then moving into an OLE.
Overall, remarkable and exciting preliminary data! We look forward to seeing full peer-reviewed publications.
And this is only the beginning. Next-generation PET tracers, PET machines with far higher sensitivities and image resolution, and biofluid analyses with better performances are ready to transform the AD landscape. Perfect timing for the introduction of disease-modifying therapies.
University of Southern California Keck School of Medicine
New Kid in Town – “everybody’s talking, everybody’s walking”
In a brief, 400-word letter to the New England Journal of Medicine last week, the FDA clearly defined its idea of substantial evidence for effectiveness in Alzheimer’s and implicitly, criteria for future approvals (Dunn et al., 2021). Simultaneously, Lilly presented donanemab pharmacodynamics and biomarkers in a way that seems to foreshadow their regulatory package for accelerated approval (Mintun et al., 2021).
The FDA letter, signed by Dunn, Stein, Cavazzoni, and for the first time by Robert Temple, reified their promotion of an amyloid plaque hypothesis as a main path to approval: If there are plaques and your drug substantially lowers plaques, then your drug is, in principle, approvable.…More
The FDA simply asserts that the substantial lowering of plaques “by a monoclonal antibody targeting aggregated amyloid is reasonably likely to predict clinical benefit.” They go on to say that current amyloid-β antibodies achieve large reductions of plaques and therefore support “a consistent relationship between degree of plaque reduction and effect on clinical end points.”
This is a rather stunning statement, as the actual evidence is to the contrary. Neither the FDA statistical review of aducanumab nor Lilly reports on donanemab back this up. Both reported correlations between changes in clinical endpoints and amyloid PET tracer values of r=-0.09 to -0.14 over 1.5 years, minuscule correlations that hardly predict or associate with anything.
Nevertheless, the promotion of a testable amyloid plaque hypothesis instead of a nuanced, complicated, and not-easily-testable amyloid cascade hypothesis, is notable advice to drug developers. Following its own 2018 draft guidelines paraphrasing in parts the 2018 ATN criteria (Jack et al., 2018), the FDA has put a simple regulatory syllogism in play:
If an anti-amyloid-β antibody markedly lowers amyloid, then Alzheimer’s disease must be improved. If tau is lowered as well, then so much the better. The postulate that lowering plaques alters disease course is easily testable as a hypothetical. In this schema clinical impairment is optional, not required for either diagnosis or a clinical endpoint. Clinical stage, however, can be specified essentially as a parallel syndrome by using Barry Reisberg’s global deterioration scale staging from 1982 (Reisberg et al., 1982). Welcome to the new normal in Alzheimer drug development.
At the AAIC donanemab emerged as the new kid in town, and the-post aducanumab era may be upon us. Donanemab’s NDA would be submitted in November, reviewed as breakthrough therapy by June, and approved in July 2022. This would be three to six months before the lecanemab and gantenerumab trials read out.
Unlike the other antibodies, donanemab has a brief and coherent story: An uneventful Phase 2 trial just meeting the primary endpoint; marked plaque reduction within six to 12 months with most in the normal range; thus far predictable, manageable edema and hemorrhage. The NDA is skimpy on sample size but still may have enough exposed patients for breakthrough review and accelerated approval. Donanemab’s approval before lecanemab and gantenerumab readouts would avert a need to show any further clinical benefit among the antibodies and ensconce plaque reduction as the surrogate clinical endpoint to beat.
At AAIC Lilly showed plasma p-tau217 correlated with plaques and tangles by PET and with cognitive decline; the dynamics of donanemab rapidly decreasing plaque; and decreased plaque associated with reduced p-tau217. They failed, however, to show an association between amyloid PET and iADRS change, showing only an r=-0.09 at 76 weeks, similar to aducanumab as well. Finally, donanemab offered the attractive idea of short-term, six- to 12-month therapy, after which you’re done, at least in terms of plaques growing back. This is appealing as we can talk about a time-limited treatment course and not indefinite treatment.
All this is beside the point, however, if these anti-amyloid-β antibodies can’t actually show clinical benefit.
(Apologies to J.D. Souther and the Eagles.)
References:
Dunn B, Stein P, Temple R, Cavazzoni P. An Appropriate Use of Accelerated Approval - Aducanumab for Alzheimer's Disease. N Engl J Med. 2021 Aug 26;385(9):856-857. Epub 2021 Jul 28 PubMed.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018 Apr;14(4):535-562. PubMed.
Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982 Sep;139(9):1136-9. PubMed.
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704. Epub 2021 Mar 13 PubMed.
University of California, San Diego
An exploration of the utility of plasma based-biomarkers in describing treatment response to amyloid-lowering monoclonal antibody treatments, lecanemab and donanemab, was reported in two presentations at AAIC 2021.
Swanson et al. reported that during initial lecanemab treatment in their proof-of-concept, placebo-controlled, Phase 2 trial, the plasma Aβ42/40 ratio increased, and this tracked to a positive treatment response during the initial placebo-controlled, double-blind treatment period. This was followed by a reversal in the ratio during a gap period when lecanemab was stopped. The reversal, with a decreasing Aβ42/40 ratio, suggested an increase in aggregation of Aβ peptides in the off-treatment period. Thereafter, a further directional reversal to an increased ratio was seen when treatment with lecanemab started again in an open-label extension (OLE). While the clinical treatment effects in the OLE are difficult to evaluate with very small numbers, and absent a control group, the movement of the plasma Aβ42/40 ratio off and on treatment is potentially of considerable importance and merits significant further investigation and replication.…More
The presentation by Mintun et al. included the findings of a strong correlation between a plasma-based p-tau217 SIMOA immunoassay and baseline amyloid PET and tau PET SUVRs. This underscores how a single blood-based biomarker might be used to predict both the likelihood of amyloid PET and of tau PET positivity, following on from the approach of C2N’s PrecivityAD test. This finding awaits further elaboration within larger and more heterogenous patient samples.
They also report that, longitudinally, the significant amyloid plaque clearance on PET is associated with a significant decline in plasma p-tau 217, with an effect size close to 24 percent from baseline with donanemab and 29 percent after adjusting for an increase in the placebo group (adjusted at 76 weeks). Interestingly, a significantly decreased p-tau217 can be seen with amyloid plaque clearance at 24 weeks. In preliminary analysis, they also report that p-tau217 reduction significantly predicts slowing of clinical decline.
Taken together, these papers underscore some of the exciting potential for plasma biomarkers to help identify treatment targets, early treatment response, and duration of treatment with what could become widely available testing. These findings need replication across amyloid-lowering therapeutics and clarification of sensitivity, specificity, and effect sizes of these changes. Much remains to be learned following on these preliminary findings, but this is very welcome attention in addressing the duration and response to amyloid-lowering treatment. This is particularly the case as new amyloid-lowering monoclonal antibodies, such as aducanumab, come into use following regulatory approval, and for which there is no guidance on duration or response to treatment.
Lund University
I think Mark Mintun’s presentation on the response of plasma pTau217 to donanemab treatment was a highlight of the AAIC 2021 meeting. We knew that anti-amyloid treatments that affect β-amyloid fibrils in the brain (e.g. aducanumab, lecanemab, bapineuzumab) do reduce p-tau levels in CSF (e.g., Salloway et al., 2014). Now, Eli Lilly shows that treatment with donanemab results in a very clear and fast reduction of plasma p-tau217 levels by approximately 25 percent. The decrease of plasma p-tau217 levels induced by donanemab correlated with the decrease in amyloid PET tracer retention, which is very encouraging to me.
I think these important results clearly indicate that p-tau217 in plasma might be used as an easily accessible and cost-effective marker that can be used to reveal drug target engagement in studies aiming at reducing the levels of β-amyloid fibrils. Further, I think we could envision a future clinical scenario where individuals who have stopped treatment with donanemab after their amyloid PET scans have normalized might be followed every six to 12 months with repeated plasma p-tau217 measurements, and when the p-tau217 levels start to increase, a new amyloid PET scan can be done to decide whether a new round of treatment with donanemab is necessary.…More
However, do we now really know if plasma p-tau217 can be used as a reliable “surrogate biomarker” to determine the clinical efficacy of new treatments? I think the Lilly results clearly show that soluble p-tau217 levels are closely associated with the levels of β-amyloid fibrils in the brain, and not only with tau aggregates; this has also been found by us and others in observational studies using neuropathology or PET to quantify the levels of β-amyloid plaques and tau aggregates (e.g., Mattsson-Carlgren et al., 2020; Mattsson-Carlgren et al., 2021; Janelidze et al., 2021). In those studies, we found that fluid p-tau levels significantly mediate the associations between measures of Aβ fibrils and tau aggregates.
However, even though these findings are congruent with the hypothesis that soluble p-tau is involved in β-amyloid-dependent formation of neocortical tau tangles, they cannot really prove that β-amyloid-induced increases of extracellular p-tau are the key driver of the subsequent build-up and spread of tau aggregates.
Therefore, I would be hesitant to already now claim that plasma p-tau217 is a “surrogate biomarker” that can be used to reliably predict future positive clinical outcomes on cognition and activities of daily living in clinical trials evaluating different classes of potential disease-modifying therapies. We simply do not yet know if the reduction of extracellular levels of soluble p-tau is an epiphenomenon merely associated with a reduction in β-amyloid fibrils in the brain, or a key event that will always lead to diminished tau aggregation, less neuronal dysfunction and reduced degeneration independently of the mechanism causing the reduced levels of extracellular p-tau.
However, if the latter turns out to be true in successful trials evaluating different classes of therapies that result in reduced extracellular p-tau levels, which, in turn, are consistently associated with positive clinical outcomes, then plasma p-tau217 would be a fantastic surrogate biomarker. It then would also be a fantastic marker that can be used for differential diagnosis, individualized prognosis, and a marker of drug target engagement for anti-amyloid therapies, as shown at AAIC by Mark Mintun and colleagues at Eli Lilly (Palmqvist et al., 2020; Palmqvist et al., 2021).
References:
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014 Jan 23;370(4):322-33. PubMed.
Mattsson-Carlgren N, Andersson E, Janelidze S, Ossenkoppele R, Insel P, Strandberg O, Zetterberg H, Rosen HJ, Rabinovici G, Chai X, Blennow K, Dage JL, Stomrud E, Smith R, Palmqvist S, Hansson O. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer's disease. Sci Adv. 2020 Apr;6(16):eaaz2387. Epub 2020 Apr 15 PubMed.
Mattsson-Carlgren N, Janelidze S, Bateman RJ, Smith R, Stomrud E, Serrano GE, Reiman EM, Palmqvist S, Dage JL, Beach TG, Hansson O. Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO Mol Med. 2021 Jun 7;13(6):e14022. Epub 2021 May 5 PubMed.
Janelidze S, Berron D, Smith R, Strandberg O, Proctor NK, Dage JL, Stomrud E, Palmqvist S, Mattsson-Carlgren N, Hansson O. Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease. JAMA Neurol. 2021 Feb 1;78(2):149-156. PubMed.
Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, Su Y, Chen Y, Serrano GE, Leuzy A, Mattsson-Carlgren N, Strandberg O, Smith R, Villegas A, Sepulveda-Falla D, Chai X, Proctor NK, Beach TG, Blennow K, Dage JL, Reiman EM, Hansson O. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020 Aug 25;324(8):772-781. PubMed.
Palmqvist S, Tideman P, Cullen N, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative, Dage JL, Stomrud E, Janelidze S, Mattsson-Carlgren N, Hansson O. Prediction of future Alzheimer's disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med. 2021 Jun;27(6):1034-1042. Epub 2021 May 24 PubMed.
USC Alzheimer’s Therapeutic Research Institute
The AAIC presentations on donanemab and lecanemab provide further support to the idea that removal of brain amyloid results in slowing of cognitive/clinical decline; this is important support for the use of aducanumab as well. Much remains to be learned, but the data to date suggests that continued therapy after normalization of amyloid may be the optimal approach to maximizing benefit. Plasma p-tau217 appears to be very valuable as an indicator of amyloid and tau pathology and as a dynamic indicator of treatment response. The treatment-related fall in p-tau217 must still be linked to clinical benefit as validation of its utility in guiding therapy. But if it can be validated, this assay will be an enormously useful tool in the drug-development process.…More
Michigan State University
I wish to kindly disagree with my friend Dr. Zetterberg regarding the value of measuring neurodegeneration markers. Our work from 2000-2008 on immunotherapy in mice has predicted the human responses well, from the clearance of parenchymal deposits to the development of ARIA-H. We found that once the amyloid is removed, cognition improves and the glial reactions resolve. Change in neurodegeneration markers parallels symptoms in multiple sclerosis treatments and this will likely also be the case in AD.
Amyloid and tau do not directly cause cognitive decline. It is the loss of synapses and neurons that does. Biomarkers indicating decreased neurodegeneration bode well for any AD therapeutic. I am confident that before and after measures of neurodegeneration markers will accelerate approval for donanemab.…More
Florey Institute of Neuroscience and Mental Health
The change in plasma p-tau217 in response to donanemab is an incredible result. I concur that this has implications not only for monitoring target engagement in clinical trials, but also for our understanding of Alzheimer’s pathophysiology. Yet, I question if this change in biomarker equates with clinical improvement.
Our enthusiasm for this drug must be tempered by the observation that donanemab accelerated brain volume loss in the TRAILBLAZER-ALZ trial, particularly after 12 months on drug (Mintun et al., 2021). This cannot be attributed to reduction in plaque volume (Ayton, 2021). While donanemab caused a modestly significant (p=0.04) initial improvement on the iADRS scale (and not four other cognitive tests), this benefit was not sustained.…More
It is possible that secondary brain damage induced by this drug neutralized any cognitive benefit over time. So, while I am intrigued by the p-tau217 and amyloid PET data, we must hold this in tension with contrary data of brain shrinkage. This is the exact opposite of what we would want to achieve with a drug for Alzheimer’s disease, and this should slow the development on donanemab until we understand more the cause of this brain atrophy.
What is more worrying is that brain shrinkage appears to be a side effect of several anti-amyloid drugs (Novak et al., 2016; Fox et al., 2005; Sperling et al., 2021; Sur et al., 2020). Is this a class effect?
This also has implications for aducanumab. I have not seen brain volume data reported or discussed for either Emerge or Engage studies in any of the documentation, meeting transcripts, and presentations released by Biogen and the FDA. On pages 167-168 of the Combined FDA and Applicant PCNS Drugs Advisory Committee Briefing Document, change in brain volume measured by MRI at week 30 and week 78 was listed as a key pharmacodynamic endpoint. Given the ambiguity in the cognitive data for this drug also, it is uncertain why there is no published evidence that the FDA considered brain volume data when adjudicating efficacy and safety of aducanumab.
Patients are being treated with this drug now, so clinicians should have all the available safety information when deciding to treat their patients. I urge Biogen to publish its findings on medRxiv. Perhaps aducanumab doesn’t cause brain shrinkage, but that would also be valuable information to release when evaluating a potential class effect of anti-amyloid drugs currently going through the regulatory process.
I declare no competing financial interests.
References:
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704. Epub 2021 Mar 13 PubMed.
Ayton S. Brain volume loss due to donanemab. Eur J Neurol. 2021 Sep;28(9):e67-e68. Epub 2021 Jul 16 PubMed.
Novak G, Fox N, Clegg S, Nielsen C, Einstein S, Lu Y, Tudor IC, Gregg K, Di J, Collins P, Wyman BT, Yuen E, Grundman M, Brashear HR, Liu E. Changes in Brain Volume with Bapineuzumab in Mild to Moderate Alzheimer's Disease. J Alzheimers Dis. 2016;49(4):1123-34. PubMed.
Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM, . Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005 May 10;64(9):1553-62. PubMed.
Sperling R, Henley D, Aisen PS, Raman R, Donohue MC, Ernstrom K, Rafii MS, Streffer J, Shi Y, Karcher K, Raghavan N, Tymofyeyev Y, Bogert J, Brashear HR, Novak G, Thipphawong J, Saad ZS, Kolb H, Rofael H, Sanga P, Romano G. Findings of Efficacy, Safety, and Biomarker Outcomes of Atabecestat in Preclinical Alzheimer Disease: A Truncated Randomized Phase 2b/3 Clinical Trial. JAMA Neurol. 2021 Mar 1;78(3):293-301. PubMed.
Sur C, Kost J, Scott D, Adamczuk K, Fox NC, Cummings JL, Tariot PN, Aisen PS, Vellas B, Voss T, Mahoney E, Mukai Y, Kennedy ME, Lines C, Michelson D, Egan MF. BACE inhibition causes rapid, regional, and non-progressive volume reduction in Alzheimer's disease brain. Brain. 2020 Dec 1;143(12):3816-3826. PubMed.
Lund University
It was reassuring to see the reduction of plasma P-tau217 already after 12 weeks of treatment. This gives us additional biomarker evidence for its efficacy.
However, since P-tau is independently associated with both amyloid and tau accumulation (Mattsson-Carlgren et al., 2021), it would be interesting in future analyses to include regression models of changes in plasma P-tau217 with both amyloid and tau PET changes to further examine what these plasma P-tau217 reductions represent. It would be interesting to see the reduction in absolute concentrations (not just presented as a relative reduction, in this case of 20 percent).
In MCI-AD and mild AD dementia, median plasma P-tau217 levels are increased approximately five to 11 times compared to amyloid-negative controls (Palmqvist et al., 2020). Hence we are likely not seeing a normalization during the trial period (such as the reduction in amyloid PET SUVR), but the results are still very promising.…More
This study also highlights the use of plasma P-tau217 as an additional marker that might be used in treatment monitoring. Nonetheless, I want to stress that biomarker results, such as plasma P-tau217, cannot replace clinically relevant outcome measures of cognition and ADL for disease-modifying treatments at the symptomatic disease stages of AD.
References:
Mattsson-Carlgren N, Janelidze S, Bateman RJ, Smith R, Stomrud E, Serrano GE, Reiman EM, Palmqvist S, Dage JL, Beach TG, Hansson O. Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO Mol Med. 2021 Jun 7;13(6):e14022. Epub 2021 May 5 PubMed.
Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, Su Y, Chen Y, Serrano GE, Leuzy A, Mattsson-Carlgren N, Strandberg O, Smith R, Villegas A, Sepulveda-Falla D, Chai X, Proctor NK, Beach TG, Blennow K, Dage JL, Reiman EM, Hansson O. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020 Aug 25;324(8):772-781. PubMed.
Make a Comment
To make a comment you must login or register.