Does Synchronizing Brain Waves Bring Harmony?
Quick Links
If enhancing memory with light and sound seems futuristic, then welcome to the future. Or so some scientists say. Results from four early stage clinical trials on mild Alzheimer’s disease were presented at the AD/PD 2021 conference, held virtually March 9 to 14. The studies used two closely related approaches to modulate brain waves. Both reportedly synchronized neuronal firing activity in the gamma frequency range, strengthened neuronal connectivity—and perhaps strengthened memory by a bit.
- Light and sound therapy boost gamma waves, neuronal connections in mild AD.
- Eight weeks of daily stimulation quieted cytokines, neuroinflammation.
- Six-month trial missed primary, but met some secondary endpoints.
Li-Huei Tsai, Massachusetts Institute of Technology, Boston, and colleagues some years ago turned light and sound into a therapy. They called it GENUS, short for gamma entrainment using sensory stimuli. Gamma brain waves, thought to be a neurophysiological correlate of attention and sensory processing, weaken in people with AD and in mouse models of amyloidosis (Dec 2016 news). Flashing a 40 Hz light at mice for an hour daily for a week enhanced and synchronized the mice’s gamma rhythms, which in turn rallied microglia to mop up plaques in their visual cortices. Adding a 40 Hz buzzing sound spread the benefits across the brain, cleared plaques and tangles, and improved the mice’s memory (Mar 2019 news; May 2019 news).
Other researchers have partially replicated these results. Sylvain Williams, McGill University, Montreal, and colleagues saw restored hippocampal gamma waves and slightly better spatial memory in amyloidosis mice after 40 Hz flashes using optogenetic stimulation, but plaques did not budge (Etter et al., 2019). Researchers led by Shuzo Sakata, University of Strathclyde, Scotland, U.K., actually saw plaques grow, also after optogenetic stimulation, but they did not assess changes in memory (Wilson et al, 2020).

Gamma Gains. In mice, light and sound induce gamma oscillations in the brain. These senses stimulate neurons, glial cells, and blood vessels, possibly clearing debris and improving synaptic function and memory. [Courtesy of Li-Huei Tsai, MIT.]
At AD/PD, Tsai presented new mouse data by her graduate student Mitchell Murdock. In 5xFAD amyloidosis mice, GENUS widened brain capillaries, increasing blood flow. It also spurred the glia-mediated lymphatic, aka “glymphatic,” system to flush the brain with cerebrospinal fluid and clear waste. How would sensory stimulation trigger CSF movement? Lined by contractile cells, blood vessels pulse not only to the rhythm of the beating heart, but also to a rhythm of their own making, and this shuffles fluid along (Iliff et al., 2013). Murdock found that after 40 Hz stimulation, arteries pulsed faster than veins, and Tsai believes this could explain the increased CSF clearing.
Could this work in people? To find out, Tsai and colleagues began a Phase 1 trial looking for data on safety, tolerability, and target engagement, i.e., entrainment. They recruited 46 healthy adults ages 18 to 85 and 26 older people with mild AD. The goal is 80 participants, but COVID temporarily stalled recruitment. Each sat in front of a panel of LED lights with a tablet in the center and a speaker that pulsed either 40 Hz light, sound, or both for one hour (see image below). The researchers played with the settings, either showing a movie on the tablet or not, and tweaking the device’s brightness and loudness, to make sure the stimulation entrained gamma waves. The researchers gauged participants’ brain activity using an electroencephalogram (EEG).

Entrainment Entertainment? Volunteers watched hundreds of flashing LED lights mounted on a four-square foot panel, and heard a high-definition sound bar buzz, both at 40 Hz. A center tablet entertained them to help them sit through an hour of this. [Courtesy of Li-Huei Tsai, MIT.]
While a one-time treatment of either light or sound alone boosted people's brain waves, the combination engaged the most brain areas in all participants. The flashing lights and humming sounds triggered no headaches, vision or hearing changes, or seizures, Tsai reported.
EEG measures mostly surface activity. Could GENUS stimulate coordinated neuronal firing deep within the brain? Tsai collaborated with Aaron Boes at the University of Iowa, Iowa City. He asked two people who had electrodes implanted into their brains because of their epilepsy to sit through a light and sound session. The researchers picked up more gamma power from the patients’ amygdalae, hippocampi, gyrus rectus, and posterior insulae during stimulation, according to Tsai’s plenary at AD/PD.
To find out what happens when people sit for this “show” regularly, the MIT scientists recruited 15 people with mild AD into a Phase 2A study. Before assigning treatment, the researchers used EEG to confirm that the optimized device settings from Phase 1 entrained gamma waves in all participants. Eight were stimulated with 40 Hz flashes and buzzes; seven received constant light and white noise as the control. Surprisingly, Tsai said, people could not tell which treatment they were receiving, possibly because they did not know what 40 Hz light looked like. “TV and computer screens are displayed at 60 Hz, so perhaps people did not expect 40 Hz to flicker that much,” she said.
Participants watched and listened for one hour daily over six or nine months, at home. To monitor compliance, the researchers recorded participants as they watched, logging how long the device was on and tracking their eye movements. Participants wore a clinical-grade Fitbit every day to track their activity and sleep. Researchers tested participants’ memory at baseline, one month, and three months, and captured structural and functional MRIs at baseline and three months. Three months into this trial, pandemic lockdowns halted data collection and delayed its follow-up timeline.
Here is what the scientists were able to glean thus far: Within one month, participants treated with flickers and hums slept better, and woke up less often during the night, than those treated with control settings. Functional MRI suggested three months of active treatment had strengthened connections in areas that process sight and in the posterior cingulate, a part of the default mode network involved in memory. Structural MRI suggested active treatment may have preserved tissue in the hippocampus, whereas control treatment did not slow atrophy. The eight people who received three months of flickers and buzz remembered more face-name pairs than the seven controls, and the difference correlated with stronger brain connectivity.
Participants in the active group were 6.4 years older than controls; they also had 5.4 more years of education than controls—the latter difference was statistically significant. Tsai said that years of education did not correlate with any outcomes, nor did it predict better performance on the face-name association test.
Regarding genetics, five treated people and four controls had one copy of ApoE4. They started the study with weaker connections in their visual networks than ApoE3 carriers, but all participants had a similar initial treatment effect regardless of their ApoE genotype.
In 2016, Tsai and her MIT colleague Ed Boyden co-founded Cognito Therapeutics in Cambridge, Massachusetts, to develop GENUS clinically. Rather than using the easel with the light panel and sound bar mounted to it, the company designed a wearable system dubbed GammaSense Stimulation (see image below). In 2018, it started three clinical trials in mild AD: Flicker, Overture, and Etude. The latter is expected to wrap up this year; the former two were presented at AD/PD.

AD Gearhead? The early version of Cognito’s apparatus combined black LED glasses with over-ear headphones. The next trials will use a sleek upgrade with a remote. [Courtesy of Annabelle Singer, Georgia Tech (left) and Kimberly Ha, KKH Advisors for Cognito Therapeutics (right).]
The Flicker Study
Also at AD/PD, James Lah, Emory University, Atlanta, and Annabelle Singer, Georgia Institute of Technology, Atlanta, presented results from the Phase 2 Flicker study, which wrapped up in February 2020. The primary endpoints were how people would tolerate daily stimulation, and if they would comply with using the device every day. The researchers recruited 10 people with mild AD from Emory’s Alzheimer’s Disease Research Center (ADRC). In their homes, five received an hour of daily double stimulation over eight weeks; the other five waited four weeks, then started their four weeks of stimulation. “This study was initially going to be placebo-controlled, but concerns about what an appropriate placebo was led us to choose a delayed start design instead,” Lah said in his presentation. This rendered Flicker an open-label trial.
First, the researchers set the flashes and buzzes from low to high intensity, asking participants to rank their comfort. Nine of the 10 tolerated the stimulation at full blast. For treatment, light and sound were then set at each person’s maximum tolerance level; for some, that was the highest intensity.
Were the strobes and sounds safe? Yes, the scientists contended at AD/PD. No one reported major side effects. That said, two volunteers experienced dizziness, two had headaches. Two people developed tinnitus, and one person's hearing loss worsened.
Participants stuck with the treatment, completing 95 percent of all sessions on average. The device automatically logged when it was on and for how long, and participants manually recorded their device usage. They stayed motivated with a study partner and weekly check-ins from a study researcher. “This was key to having such high adherence,” Singer noted. Nine chose to continue treatment in a 10-month, open-label extension.

Flicker In, EEG Out. A research volunteer receives 40Hz sensory treatment through a GENUS prototype apparatus while her brain activity is being recorded simultaneously via an EEG cap. [Courtesy of Annabelle Singer, Georgia Tech.]
Did their brain waves become entrained? The scientists recorded EEGs at baseline and after four or eight weeks of neuromodulation. At AD/PD, Lah reported that gamma waves matched the 40 Hz stimuli during each timepoint in an average of 49 of the 64 brain areas hooked up to the EEG. Gamma power stayed unchanged after four weeks of stimulation, and then it weakened after eight weeks. This stumped the researchers. “We expected to see stronger gamma waves, but the fact that we saw a difference in gamma activity at all was important” Lah said.
Singer and colleagues tracked brain connectivity using functional MRI captured at baseline, four, and eight weeks of treatment. Here, too, nothing changed after four weeks of treatment. After eight weeks, neuronal connections strengthened between the posterior cingulate and precuneus, two areas in the default network prone to disruption due to amyloid plaque buildup in people with AD (Sept 2020 news; Aug 2009 news).
As for neuroinflammation, Singer had recently published that, in wild-type mice, neurons jiving at 40 Hz thanks to gamma visual stimulation release cytokines, such as interleukin-4 and -6, that activate microglia to vacuum up debris (Garza et al., 2020).
How about in people? In Flicker, scientists measured 77 different cytokines in the CSF before and after eight weeks of daily stimulation. At AD/PD, Singer reported that about two-thirds decreased after eight weeks (see image below). She highlighted two in particular: macrophage inflammatory protein-1β (MIP-1β), which controls microglia, and TNF-like weak inducer of apoptosis (TWEAK), which upregulates NF-κB and other cytokines (e.g., Samidurai et al., 2020).
Other groups have shown that inhibiting TWEAK increases synaptic signaling in hippocampal slices from an amyloidosis mouse model, decreases microglial activation in an ALS mouse model, and attenuates neurological symptoms in a model of the auto-immune disease lupus erythematosus (Nagy et al., 2021; Bowerman et al., 2015; Wen et al., 2016). Singer thinks TWEAK may be regulating microglia and other cytokines. “It could be a key knob controlling many other immune factors,” she said.

Shifting Cytokines. After eight weeks of neuromodulation, CSF levels of two-thirds of 77 cytokines measured were lower. Some of them stimulate astrocytes (TGF-α), microglial proliferation (IL-5), and microglial motility (MIP-1β). Among the cytokines raised, some promote neural and glial cell differentiation (LIF), flag neurovascular injury (MMP-10), and recruit neutrophils (IL-8). [Courtesy of Annabelle Singer, Georgia Tech.]
What about amyloid and tau? In Flicker, amyloid PET, CSF Aβ, total tau, and phospho-tau 181 stayed the same after eight weeks. “Perhaps nothing changed because it was such a short and small study,” Singer said.
The Overture Study
This Phase 2A trial evaluated daily gamma stimulation for six months. In back-to-back presentations at AD/PD, Cognito’s Thomas Megerian and Suzanne Hendrix, Pentara Corporation, Salt Lake City, gave updates on the trial, which is slated to end in August 2021. It enrolled 76 people aged 50 and older across the U.S. who had mild to moderate AD; two withdrew before starting treatment. Under the watch of a caregiver at home, all participants wore the GammaSense device for one hour every day for six months. For 46 of them, the device emitted 40 Hz sound and light as active treatment; for 28, it was set to “sham” settings, which the company did not specify. To track compliance, the device records when and for how long it is used daily.
The control group was older and scored worse on memory tests, on average. Hendrix said she accounted for those imbalances during data analysis by including baseline memory test scores for each participant.
According to Megerian, participants tolerated the treatment well, and it was safe overall. That said, echoing the Flicker finding, people on active treatment complained of tinnitus more than those exposed to sham settings. In each group, 28 percent dropped out early by withdrawing their consent or having an adverse reaction that led them to quit. One of the three participants who left the treatment group due to an adverse reaction did so because they developed tinnitus. Others grew tired of the time commitment—people with moderate AD had a tougher time sitting for the daily hour than those with mild AD. Most dropouts happened within the first two months. “Once a participant hit two or three months, they stuck with it,” Megerian added. Forty-four of the 53 participants who finished the trial continued into a one-year open-label extension.
The researchers tracked brain changes with structural MRI before and after six months of treatment. Active treatment preserved 61 percent more tissue throughout the brain than control. “This was unexpected and very interesting,” Tsai remarked. Hendrix agreed. “It is fairly unusual to see large changes in the whole brain volume,” she said.
In AD, the hippocampus usually shows signs of atrophy early on, and in the Phase 2A study also reporting data at AD/PD (see above), GENUS appeared to protect this deep-brain area. In the larger Overture study, the hippocampi in active treatment and sham groups shrunk at similar rates, Hendrix reported.
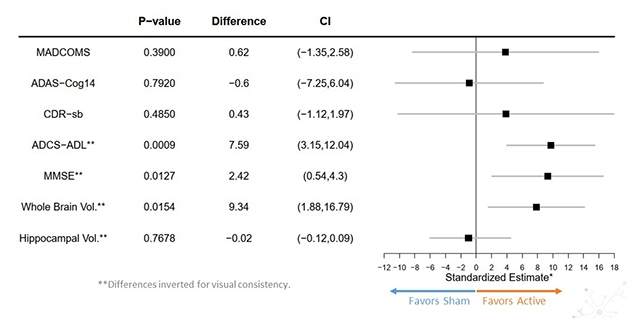
Missed Primary. The cognitive clinical batteries ADAS-Cog14, CDR-sb, and MADCOMS remained unchanged after GENUS; functional and mental state tests showed less slippage. GENUS preserved whole brain volume, but not in the hippocampus specifically. [Courtesy of Suzanne Hendrix, Pentara Corporation, and Kimberly Ha for Cognito Therapeutics.]
What about cognition, though? At baseline, three, and six months of treatment, the participants sat for the ADAS-Cog14, CDR-sb, MADCOMS (a combination of questions from the two previous batteries), ADCS-ADL, and MMSE. The three former constituted the primary, the latter two, secondary endpoints. Memory and cognition did not differ between participants on active or sham treatment, with all three primaries falling far short of statistical significance (see image above). In contrast, daily functioning and mental status declined 84 and 83 percent more slowly in 40Hz-treated participants as measured by ADCS-ADL and MMSE, respectively. This was statistically significant.
Why some outcome measures showed no difference and some did puzzled researchers. “We need to carefully consider what aspects of cognition to measure,” Hendrix said. Future trials might explore how strengthening brain connections affects cognitive function more broadly, not just memory, Megerian said. Hendrix proposed comparing ADAS-Cog scores to measures of processing speed, executive function, or attention. “These tasks are not covered well by the ADAS-Cog, and we might be seeing effects in those areas,” she said. Megerian agreed. GENUS focuses on increasing broad neuronal connectivity that may improve functions not well examined by the ADAS-Cog, he said.
Hendrix wondered if GammaSense might help certain patients more than others, such as people with low or high brain amyloid loads. The researchers collected amyloid PET scans, CSF, and blood samples in this trial, but have yet to analyze them.
Even though Overture missed its primary endpoint, the researchers were hopeful. “The most important thing is that we see evidence of target engagement and some changes in imaging biomarkers and memory,” Lah said, adding, “These small studies warrant additional larger studies.”
Cognito is planning to start a Phase 3 trial later this year (company press release). Megerian said Cognito will also evaluate GENUS in other conditions, such as Down’s syndrome.—Chelsea Weidman Burke
References
News Citations
- Flashy Treatment Synchronizes Neurons, Lowers Aβ in Mice
- Flash! Beep! Gamma Waves Stimulate Microglia, Memory
- Gamma Waves Synchronized by Light: Good for Synapses, Memory?
- In Preclinical Alzheimer’s, Learning Falters Before Memory
- BOLD New Look—Aβ Linked to Default Network Dysfunction
Research Models Citations
Paper Citations
- Etter G, van der Veldt S, Manseau F, Zarrinkoub I, Trillaud-Doppia E, Williams S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer's disease mouse model. Nat Commun. 2019 Nov 22;10(1):5322. PubMed.
- Wilson CA, Fouda S, Sakata S. Effects of optogenetic stimulation of basal forebrain parvalbumin neurons on Alzheimer's disease pathology. Sci Rep. 2020 Sep 22;10(1):15456. PubMed.
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013 Nov 13;33(46):18190-9. PubMed.
- Garza KM, Zhang L, Borron B, Wood LB, Singer AC. Gamma Visual Stimulation Induces a Neuroimmune Signaling Profile Distinct from Acute Neuroinflammation. J Neurosci. 2020 Feb 5;40(6):1211-1225. Epub 2019 Dec 23 PubMed.
- Samidurai M, Tarale P, Janarthanam C, Estrada CG, Gordon R, Zenitsky G, Jin H, Anantharam V, Kanthasamy AG, Kanthasamy A. Tumor Necrosis Factor-Like Weak Inducer of Apoptosis (TWEAK) Enhances Activation of STAT3/NLRC4 Inflammasome Signaling Axis through PKCδ in Astrocytes: Implications for Parkinson's Disease. Cells. 2020 Aug 4;9(8) PubMed.
- Nagy D, Ennis KA, Wei R, Su SC, Hinckley CA, Gu RF, Gao B, Massol RH, Ehrenfels C, Jandreski L, Thomas AM, Nelson A, Gyoneva S, Hajós M, Burkly LC. Developmental synaptic regulator, TWEAK/Fn14 signaling, is a determinant of synaptic function in models of stroke and neurodegeneration. Proc Natl Acad Sci U S A. 2021 Feb 9;118(6) PubMed.
- Bowerman M, Salsac C, Coque E, Eiselt É, Deschaumes RG, Brodovitch A, Burkly LC, Scamps F, Raoul C. Tweak regulates astrogliosis, microgliosis and skeletal muscle atrophy in a mouse model of amyotrophic lateral sclerosis. Hum Mol Genet. 2015 Jun 15;24(12):3440-56. Epub 2015 Mar 12 PubMed.
- Wen J, Chen CH, Stock A, Doerner J, Gulinello M, Putterman C. Intracerebroventricular administration of TNF-like weak inducer of apoptosis induces depression-like behavior and cognitive dysfunction in non-autoimmune mice. Brain Behav Immun. 2016 May;54:27-37. Epub 2015 Dec 23 PubMed.
Other Citations
External Citations
Further Reading
Papers
- Strüber D, Herrmann CS. Modulation of gamma oscillations as a possible therapeutic tool for neuropsychiatric diseases: A review and perspective. Int J Psychophysiol. 2020 Jun;152:15-25. Epub 2020 Mar 30 PubMed.
- Adaikkan C, Tsai LH. Gamma Entrainment: Impact on Neurocircuits, Glia, and Therapeutic Opportunities. Trends Neurosci. 2020 Jan;43(1):24-41. Epub 2019 Dec 10 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Picower Institute of MIT
Many thanks for putting together such a comprehensive review of GENUS work reported at ADPD. I’d like to add that three additional groups have independently replicated our preclinical data on GENUS.
Park et. al. sought to determine whether treadmill exercise and 40Hz light flicker could provide a combined benefit for 3xTg Alzheimer’s model mice. They found, after a three-month regimen, that it did: “The combination of exercise and 40-Hz light flickering exposure was most effective in reducing Aβ and tau levels.” They found therapeutic effects regarding cell death, cell differentiation, and neurogenesis in the hippocampus, and regarding cognitive impairment such as spatial learning, memory, and long-term memory (Park et al., 2020).
In a mouse model of cerebral ischemia, Zheng et. al. found that 40Hz light flicker restored hippocampal gamma rhythm power and, coupling with theta rhythms, provided protection against neurodegeneration. “These results support a causal relationship between CA1 slow gamma and cognitive dysfunctions in the ischemic brain,” the authors wrote (Zheng, et al., 2020).
Bobola et. al. attempted to determine whether using 40Hz-focused ultrasound, rather than sensory stimulation, would affect Aβ plaques in specifically targeted regions of the brains of 5XFAD Alzheimer’s model mice. As our lab did using light flicker, Bobola’s group achieved microglial activation and a 50 percent reduction in plaques: “Our results compare to those of Iaccarino et al. (2016) but throughout the area of ultrasound-exposed brain” (Bobola et al., 2020).
References:
Park SS, Park HS, Kim CJ, Kang HS, Kim DH, Baek SS, Kim TW. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer's disease. Alzheimers Res Ther. 2020 May 20;12(1):62. PubMed.
Zheng L, Yu M, Lin R, Wang Y, Zhuo Z, Cheng N, Wang M, Tang Y, Wang L, Hou ST. Rhythmic light flicker rescues hippocampal low gamma and protects ischemic neurons by enhancing presynaptic plasticity. Nat Commun. 2020 Jun 15;11(1):3012. PubMed.
Bobola MS, Chen L, Ezeokeke CK, Olmstead TA, Nguyen C, Sahota A, Williams RG, Mourad PD. Transcranial focused ultrasound, pulsed at 40 Hz, activates microglia acutely and reduces Aβ load chronically, as demonstrated in vivo. Brain Stimul. 2020 Jul - Aug;13(4):1014-1023. Epub 2020 Apr 1 PubMed.
This is a good summary article.
I suggest readers look at "Transcranial Near Infrared Light Stimulations Improve Cognition in Patients with Dementia" by Damir Nizamutdinov et al.
References:
Nizamutdinov D, Qi X, Berman MH, Dougal G, Dayawansa S, Wu E, Yi SS, Stevens SB, Huang JH, Scott B, White Health. Transcranial Near Infrared Light Stimulations Improve Cognition in Patients with Dementia. Aging and Disease, March 21, 2021.
Make a Comment
To make a comment you must login or register.