Plaques, Tangles Throw Off the Brain’s Rhythms
Quick Links
Brain rhythms change their tune in people with Alzheimer’s disease, and they do so to the beat of Aβ and tau, according to a study published in Science Translational Medicine on March 11. Researchers led by Keith Vossel and Srikantan Nagarajan at the University of California, San Francisco, reported that while alpha wave synchrony collapses in certain regions of the brain, delta-theta waves synchronize more tightly in others. Where in the brain the alpha synchrony fell apart correlated with where tau tangles were. It also differed among clinical subtypes of AD. In contrast, heightened delta-theta synchrony occurred in regions burdened by either Aβ or tau. The findings show that Aβ and tau degrade the function of the brain’s circuitry in different ways in AD, and suggest that the electrical readouts might serve as functional biomarkers.
- In people with Alzheimer’s, alpha rhythms fell out of step, whereas delta-theta rhythms became more synchronous.
- Regional alpha hyposynchrony mapped to tangles, differed among AD clinical subtypes.
- Delta-theta hypersynchrony aligned with Aβ and tau pathology.
“[The findings] provide further support for the notion that the cellular and molecular changes in AD converge at the level of circuits,” wrote Samuel Harris and Marc Aurel Busche of University College London in a joint comment to Alzforum. The study also points to novel diagnostic and therapeutic approaches, they added.
“This study adds to the literature on the value of neurophysiological biomarkers on top of other more established biomarkers, e.g. Aβ, tau, and neurodegeneration markers, in better diagnosing, classifying, and predicting trajectories in Alzheimer’s disease,” wrote Tarek Rajji, University of Toronto, to Alzforum.
Far from a mass of neurons firing at random, the brain is an exquisitely organized mesh of functional circuitry. The coordinated firing of clusters of neurons throughout the brain yields electrical pulses of different frequencies, ranging from slow delta-theta rhythms that beat at 2 to 8 Hz, to moderate 8 to 12 Hz alpha waves, to the rapid gamma waves that flutter at 30 to 100 Hz.
Using electroencephalography (EEG) or magnetoencephalography (MEG) to detect these rhythms through the scalp, researchers have reported changes in people with AD—alpha waves weaken, delta-theta waves swell (Osipova et al., 2005; Stam et al., 2006; Lizio et al., 2011). Functional MRI studies, which gauge neuronal activity indirectly by measuring local blood oxygen levels, also point to disturbances in the brain networks of people with AD (Mar 2004 news; Aug 2009 news). However, exactly where these electrical disturbances arise, and how they relate to the regional distribution and severity of AD pathology in the brain, remain tough questions to answer.
First author Kamalini Ranasinghe and colleagues combined electrical recordings with multimodal brain imaging. First, they charted patterns of neuronal synchrony, using magnetoencephalography imaging, MEGI for short. This technique combines MEG readings from 275 scalp sensors with individualized structural MRI scans to measure brain rhythms with improved spatial and temporal resolution. Essentially, for each of about 2,000 voxels in the brain, an algorithm calculates how closely brain rhythms sync up with those in the rest of the brain.
Compared with 20 healthy controls, 60 people diagnosed with probable AD or MCI due to AD had less synchronous alpha rhythms and hypersynchronous delta-theta waves. Alpha hyposynchrony predominated in the left inferior temporal, left posterior parietal, and bilateral occipital regions, while delta-theta hypersynchrony occurred in more anterior regions, including the frontal and parietal cortices and the right temporal cortex.
Curiously, regions with most alpha hyposynchrony differed among people with distinct clinical subtypes of AD. Among 30 people with the typical amnestic, dysexecutive type of AD, alpha waves fell most out of step in the posterior parietal and occipital cortices, while among 15 participants with the logopenic variant of primary progressive aphasia, the left posterior temporal cortex was most affected. People with the posterior cortical atrophy variant of AD had the poorest alpha synchrony in the occipital cortices, with their right hemispheres doing worst.
In contrast with alpha synchrony, regional patterns of delta-theta hypersynchrony did not differ among clinical AD subtypes.

Synchrony subtypes. Patterns of alpha hyposynchrony (left) differed significantly among clinical subtypes of AD, whereas the distribution of delta-theta hypersynchrony (right) did not. [Courtesy of Ranasinghe et al., Science Translational Medicine, 2020.]
In a subset of 12 AD patients who had tau PET scans, waning alpha synchrony also appeared to coincide with tangles. Regions most afflicted by tau pathology—the posterior and inferior temporal cortices and left posterior parietal cortex—also emitted alpha waves most out of sync with the rest of the brain. Alpha hyposynchrony did not align with patterns of Aβ deposition (see image above). In contrast, delta-theta hypersynchrony co-localized with Aβ deposition as well as tau tangles. Either pathology alone boosted it, but in regions heavily burdened by Aβ and tau, the net effect was a lowering of delta-theta synchrony.
“This suggests that delta/theta synchrony reflects an interaction between Aβ and tau pathologies, and it will thus be fascinating to confirm in longitudinal studies whether delta/theta hypersynchrony at early stages of AD, transitions to hyposynchrony in late stages, and may be a biomarker of disease progression,” wrote Harris and Busche. This would dovetail with data from mouse models reported by Busche and others, in which Aβ triggered an uptick in neuronal signaling, while tau silenced it. When put together, tau’s silencing effect won out (Dec 2018 conference news).
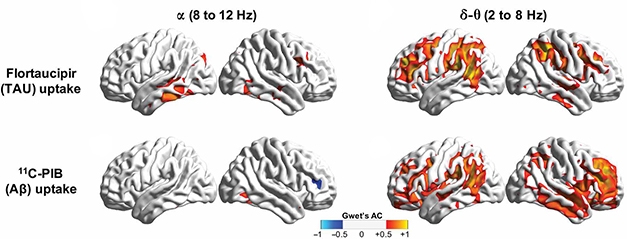
In Sync with Aβ, Tau. Alpha hyposynchrony (left) more closely aligned with tau PET (top) than with Aβ PET (bottom). Delta-theta hypersynchrony (right) correlated with both tangles and plaques. [Courtesy of Ranasinghe et al., Science Translational Medicine, 2020.]
Ranasinghe noted that while delta-theta rhythms associate with excitatory neurons, alpha rhythms reflect inhibitory circuits. Essentially, alpha rhythms gate the flow of information in the brain, she said. Alpha hyposynchrony could release the brakes on excitatory circuits, leading to hyperactivity. That alpha synchrony slips in people with AD meshes with previous reports of hyperactivity in their default mode network, she added.
Finally, Ranasinghe and colleagues reported that alpha hyposynchrony, but not delta-theta hypersynchrony, associated with poorer cognition, as assessed by MMSE scores. Together, the findings place alpha hyposynchrony most proximal to the business end of AD, i.e., tau pathology and cognitive decline. Ranasinghe noted that while only three to four people with each clinical subtype of AD underwent tau PET scans, the results suggest that differences in the distribution of alpha hyposynchrony among the clinical subtypes could be dictated by tau deposition patterns.
“The results confirm previous findings that AD has clinical variations and that these are linked to concordant anatomical differences in the distribution of neurofibrillary tauopathy,” wrote Marsel Mesulam and Emily Rogalski of Northwestern University, Chicago, in a comment to Alzforum. They added that the MEGI findings mesh with those of their recent resting-state fMRI study, in which unique perturbations in network connectivity cropped up in people with amnestic versus aphasic symptoms (Martersteck et al., 2020).
The study adds to mounting evidence implicating offbeat brain rhythms in cognitive decline. While animal studies suggest that stimulating the brain with gamma waves can help mop up Aβ plaques and boost memory, a recent study in humans found that getting theta rhythms back on track improved working memory (Dec 2016 news; Mar 2019 news; Apr 2019 news).
Ranasinghe did not measure fast gamma waves in this study because these waves are not detected in the resting state; they only pop up during tasks. However, she noted that slower alpha and theta waves are clearly tied to gamma waves through a phenomenon called phase-amplitude coupling, in which the amplitude of gamma waves ebb and flow with the phase of slower theta or alpha waves.
In a joint comment to Alzforum, Li-Huei Tsai and Chinnakkaruppan Adaikkan of Massachusetts Institute of Technology noted that the changes in theta and alpha coherence in people with AD fit nicely with previous studies in rodents, which reported that theta/alpha coherence is enhanced during spatial memory tasks (Jones and Wilson, 2005).
“Future longitudinal studies may reveal how and why disruptions in network synchrony evolve as AD pathogenesis progress,” they added. “Overall, these findings point to the idea that targeting network synchrony early on in the disease progression may offer benefits.”
Future studies will track changes in brain rhythms along the spectrum of AD, Ranasinghe said. With a better understanding of how brain rhythms change throughout the course of the disease, these electrical signals may one day serve as sensitive markers of progression or for specific subtypes of AD. This would help researchers track specific cognitive deficits, such as memory or language, in the course of a clinical trial.
Rajji agreed. “The findings support the potential use of these neurophysiological markers in identifying different aspects of the AD syndromes for clinical classification and treatment interventions,” he wrote.—Jessica Shugart
References
News Citations
- Network Diagnostics: "Default-Mode" Brain Areas Identify Early AD
- BOLD New Look—Aβ Linked to Default Network Dysfunction
- Tau Silences, Aβ Inflames; Hitting Excitatory Synapses Hardest
- Flashy Treatment Synchronizes Neurons, Lowers Aβ in Mice
- Flash! Beep! Gamma Waves Stimulate Microglia, Memory
- Resetting Brain Rhythms Gives Working Memory a Brief Boost
Paper Citations
- Osipova D, Ahveninen J, Jensen O, Ylikoski A, Pekkonen E. Altered generation of spontaneous oscillations in Alzheimer's disease. Neuroimage. 2005 Oct 1;27(4):835-41. PubMed.
- Stam CJ, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JP, de Munck JC, van Dijk BW, Berendse HW, Scheltens P. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer's disease. Neuroimage. 2006 Sep;32(3):1335-44. PubMed.
- Lizio R, Vecchio F, Frisoni GB, Ferri R, Rodriguez G, Babiloni C. Electroencephalographic rhythms in Alzheimer's disease. Int J Alzheimers Dis. 2011;2011:927573. PubMed.
- Martersteck A, Sridhar J, Rader B, Coventry C, Parrish T, Mesulam MM, Rogalski E. Differential neurocognitive network perturbation in amnestic and aphasic Alzheimer disease. Neurology. 2020 Feb 18;94(7):e699-e704. Epub 2020 Jan 22 PubMed.
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005 Dec;3(12):e402. Epub 2005 Nov 15 PubMed.
Further Reading
Papers
- de Haan W, Stam CJ, Jones BF, Zuiderwijk IM, van Dijk BW, Scheltens P. Resting-state oscillatory brain dynamics in Alzheimer disease. J Clin Neurophysiol. 2008 Aug;25(4):187-93. PubMed.
- Stam CJ. Use of magnetoencephalography (MEG) to study functional brain networks in neurodegenerative disorders. J Neurol Sci. 2010 Feb 15;289(1-2):128-34. PubMed.
Primary Papers
- Ranasinghe KG, Cha J, Iaccarino L, Hinkley LB, Beagle AJ, Pham J, Jagust WJ, Miller BL, Rankin KP, Rabinovici GD, Vossel KA, Nagarajan SS. Neurophysiological signatures in Alzheimer's disease are distinctly associated with TAU, amyloid-β accumulation, and cognitive decline. Sci Transl Med. 2020 Mar 11;12(534) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University College London
University College London
This paper is a significant step forward in our understanding of how AD pathology quantitatively manifests at the circuit level. Not only does it confirm and extend recent evidence of altered circuit function in early AD, it also, critically, supports related data obtained by us and others in translational models. Importantly, the data presented by Ranasinghe et al. suggests that circuit-based functional readouts can be leveraged to differentially profile AD phenotypes and acts as biomarkers of underlying pathological changes.
It will be of great interest to the field whether these results are also applicable to larger cohorts, and in particular to other related neurodegenerative disorders. For example, different tauopathies exhibit distinctive pathoprogression properties, and it would be fascinating to determine whether alpha band hyposynchrony, which co-localised to areas of increased tau in the current paper, is also able to discriminate between these variants. A related question pertains to the, as yet unclear, mechanism(s) by which tau engenders hyposynchrony. Tau-related neuronal hypoactivity, as reported by us and others, likely plays a key role in destabilizing the overlying circuit, and further work will elucidate whether nicotinic acetylcholine receptors, which have been linked to altered alpha oscillations and tau pathology, may also be candidate foci.
In contrast, the current paper reports that delta/theta hypersynchrony was unable to differentiate between AD phenotypes and co-localized to both Ab and tau deposition. Importantly, voxel-wise general linear model analysis revealed that differential uptake of both peptides was associated with modulation of delta/theta synchrony, such that areas with both high Ab and tau uptake were associated with hyposynchrony, while others in which Ab or tau uptake were high when tau or Ab were low, respectively, were linked to hypersynchrony. This suggests that delta/theta synchrony reflects an interaction between Ab and tau pathologies, and it will thus be fascinating to confirm in longitudinal studies whether delta/theta hypersynchrony at early stages of AD transitions to hyposynchrony at late stages, and if synchrony may be a biomarker of disease progression. It will also be interesting to determine whether the recently reported improvements in Ab peptide burden evoked by gamma-frequency stimulation reflects a rectification of altered low-frequency synchronization (such as in the frequency bands reported here), given known cross-frequency coupling processes.
In summary, the data presented by Ranasinghe et al. provide further support to the notion that the cellular and molecular changes in AD converge at the level of circuits. This study is an important advance in a growing body of literature describing circuit level changes in AD, and it signposts new horizons for novel diagnostic and therapeutic approaches.
Northwestern University
University of Chicago
Ranasinghe et al. report that different clinical phenotypes of Alzheimer’s disease (AD) are associated with different patterns of EEG synchronization abnormalities.
The magnetoencephalographic recordings, molecular imaging, and multimodal analyses in this paper are elegant. The results confirm previous findings that AD has clinical variations and that these are linked to concordant anatomical differences in the distribution of neurofibrillary tauopathy (Gefen et al., 2012). In keeping with this heterogeneity, perturbations of network connectivity in AD have been shown to be more profound in the hippocampus when the phenotype is amnestic, and in the inferior frontal gyrus (Broca’s area) when the phenotype is aphasic (Martersteck et al., 2020).
Together with the findings of Ranasinghe et al., we can now conclude that the anatomy of AD pathology and its clinical manifestations are not entirely determined by the cellular and molecular biology of the disease and that there are critical interactions with patient-specific factors and co-morbidities that remain to be identified.
References:
Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, Wieneke C, Bigio E, Geula C, Mesulam MM. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012 May;135(Pt 5):1554-65. PubMed.
Martersteck A, Sridhar J, Rader B, Coventry C, Parrish T, Mesulam MM, Rogalski E. Differential neurocognitive network perturbation in amnestic and aphasic Alzheimer disease. Neurology. 2020 Feb 18;94(7):e699-e704. Epub 2020 Jan 22 PubMed.
Massachusetts Institute of Technology
Picower Institute of MIT
Based on the findings that theta/alpha (4-12 Hz) coherence is enhanced between brain areas during spatial memory tasks in rodents, one could hypothesize that disruptions in theta/alpha synchrony or coherence may occur in situations of memory dysfunction (Jones and Wilson, 2005). The current study nicely shows that reduced 8-12 Hz synchrony correlates not only with cognitive dysfunction, but also in pathological tau deposition in AD. In addition, the authors report aberrant 2-8 Hz synchrony in AD. Future longitudinal studies may reveal how and why disruptions in network synchrony evolve as AD pathogenesis progress. It is worth noting that a previous longitudinal study has already shown slow-wave oscillations are also affected in AD (Lucey et al., 2019). Overall, these findings point to the idea that targeting network synchrony early on in disease progression may offer benefits.
References:
Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005 Dec;3(12):e402. Epub 2005 Nov 15 PubMed.
Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TL, Holtzman DM. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med. 2019 Jan 9;11(474) PubMed.
University of Texas Southwestern Medical Center
This is an elegantly designed study using multidomain imaging to better understand network dysfunction in Alzheimer’s disease and their relationships to phenotype. At a general level, this study adds to the literature on the value of neurophysiological biomarkers on top of other more established biomarkers, e.g. Aβ, tau, and neurodegeneration biomarkers, in better diagnosing, classifying, and predicting trajectories in Alzheimer’s disease.
Their multidomain imaging approach allowed the authors to discover some interesting findings. The specificity of co-localization and association between alpha hyposynchrony and tau, but not Aβ, deposition is very interesting and points toward specific mechanisms underlying these different neurophysiological markers. This idea is also supported by the fact that the authors found an association and co-localization between delta-theta hypersychrony and tau and Aβ deposition.
Another exciting finding is that different AD phenotypes had different anatomical profiles of alpha hyposynchrony but a common delta-theta hypersynchrony profile. This finding supports the potential use of these neurophysiological markers in identifying different aspects of the AD syndromes for clinical classification and treatment interventions.
The work reported in this paper is very promising for the field of neurophysiology in AD. It warrants attempts of replication with larger sample sizes and across multiple sites. It also advances further the agenda for using these neurophysiological markers to personalize interventions that can be moderated by these markers or that can target them directly.
Alzheimer Center Amsterdam; Head EQT Life Sciences Dementia Fund
VU University Medical Center
The general message that a noninvasive neurophysiological technique can provide that much relevant information on molecular AD pathophysiology based on just one minute of data is quite baffling, and illustrates the growing relevance of MEG for clinical and research purposes in AD. Alpha (8–12 Hz) hyposynchrony has been described before in AD in EEG and MEG studies, but we haven’t seen such an evident spatial relation with specific AD-variants yet.
The fact that resting-state, source-space (i.e., sensor space signals are used to reconstruct the activity sources, including deeper regions) MEG data is used, is in line with literature and our own experience that this approach produces robust and relevant results. So there seems to be no need for specific cognitive tasks or evoked potentials, which can be difficult to assess and interpret in this patient group. In addition, this study supports the view that functional connectivity analysis seems to add new information to the more established, local analysis (e.g., power spectral density).
We agree with the suggestion that modulating spontaneous brain rhythms may open up new therapeutic options (and are currently pursuing this aim); oscillatory behavior may be more “downstream” from AD pathology than synapse function, but that does not automatically make it a less effective potential treatment target. Also, involving not just local activity but connectivity patterns as well will lead to more rational strategies when modulating highly interconnected human brain networks.
While we consider this an important study, there are some limitations. For functional connectivity analysis, the “imaginary coherence” coupling measure was used. There are many connectivity measures, and all of them have different strong and weak points, so this particular choice may have had consequences for the interpretation of the results. A confirmation of these results with other coupling measures could strengthen the message. A further limitation is that the set of patients that received all modalities (MEG, tau-PET and Aβ-PET) is rather small (n=12), as the authors argue themselves. One final remark on the interpretation of the results: the overlapping spatial patterns of abnormal synchrony and amyloid/tau deposition are explained as a modulation of the latter on the former. However, since there are also reports of neuronal firing rates actually driving protein deposition, the causal chain in this relationship is still open for debate: e.g., a bidirectional, pathological influence is conceivable.
IRCCS FBF
This paper highlights some striking observations:
1) Alpha rhythm hyposynchrony is specifically related to both Tau burden and cognitive state;
2) The left hemisphere is the main affected brain region in Alzheimer’s disease;
3) Delta/theta synchrony has a double, counterintuitive, behavior: earlier hyper-, later hyposynchrony, suggesting an inverse relationship with Aβ/Tau burden.
It should be considered that the so-called alpha rhythm (8-12 Hz) is not a unitary phenomenon. Rather, it encompasses different oscillatory components, so that we can describe at least slow and high alpha rhythms, both with different functional tasks and neural generators. If we could detect a different synchrony of the sub-alpha components, computed in an individual way, we could shed light on the subtle connectivity damage in AD network and find a specific biomarker of the disease.
Make a Comment
To make a comment you must login or register.