CONFERENCE COVERAGE SERIES
Chronic Traumatic Encephalopathy
Lou Ruvo Center for Brain Health, Las Vegas, Nevada, U.S.A.
30 September – 01 October 2012
CONFERENCE COVERAGE SERIES
Lou Ruvo Center for Brain Health, Las Vegas, Nevada, U.S.A.
30 September – 01 October 2012
When autopsies of football stars and wrestlers who had committed suicide touched off a storm of media coverage some years ago, the initial story was one of concussions putting athletes at risk for Alzheimer’s disease. Since then, however, the story has taken a sharp turn. Prompted by striking brain pathology in both contact sport athletes and military veterans, scientists are now defining a new disease. Called chronic traumatic encephalopathy (CTE), the underlying concept envisions a massive tauopathy that spreads from the site of impact throughout the brain during the months and years after hits to the head. In other words, a progressive disease that stands apart from the known manifestations of traumatic brain injury (TBI). On 30 September to 1 October 2012, at the first research conference dedicated exclusively to CTE, scientists promulgated the idea that, of the estimated 1.7 million people who sustain mild TBIs in the U.S. every year, an untold number do not recover, nor do they live with the chronic, stable impairment that is sometimes called post-concussion syndrome. Instead, they develop a discrete secondary tauopathy that worsens with age and eventually leads to dementia or parkinsonism if the person survives long enough. Emerging research hints that CTE may self-propagate from cell to cell, as do other tauopathies.
In this way, CTE would be a new neurodegenerative disease. CTE is distinct from Alzheimer’s, Parkinson’s, frontotemporal dementia (FTD), or amyotrophic lateral sclerosis (ALS), though individual cases can overlap with either of these. “CTE is a debilitating disease found in people with a history of repeated brain trauma,” Vice Admiral Regina Benjamin, the U.S. Surgeon General, told a unique gathering of scientists and advocates at the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, Nevada. “CTE can start months or years after brain trauma has occurred, either in contact sports, military service, veterans, or perhaps even partner violence, shaken baby syndrome, motor vehicle accidents, and other circumstances of life in the U.S.,” the nation’s top physician said in Las Vegas.
Lou Ruvo Center for Brain Health, Las Vegas, Nevada Image courtesy of Gabrielle Strobel

Cleveland Clinic
After summarizing what is known about CTE, the Las Vegas gathering exchanged the latest information from ongoing research projects on boxers, more recent neuropathology, and CTE transmission in mice (see Part 2, Part 3, and Part 4 of this series). Then they articulated current challenges. For example, how can researchers, and eventually doctors in community practice, distinguish TBI from CTE? On this, progression is the key difference, so much so that the name might eventually change. “It may eventually be 'chronic progressive encephalopathy.' That’s because there are many people who have a stable encephalopathy following trauma. They probably do not have a progressive tau disorder,” Cumming said.
The incidence and prevalence of CTE are unknown at this point, even though the military in particular is concerned it may be high. “I believe we have a very large population of people who may be at the beginning of what we are talking about here,” said Captain Paul Hammer, who directs the Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury. The Department of Defense’s estimate of mild TBIs has risen steeply since 2005.
To obtain epidemiological data, scientists need to know for sure who is a case. They need a consistent diagnosis—ideally a clinical/cognitive one anchored to the underlying disease process by biomarkers—to delineate the progressive disorder from TBI. At the Lou Ruvo conference, Stern introduced draft consensus diagnostic criteria for CTE that were inspired by recent advances in Alzheimer’s (see Part 5). In that disease, a 25-year-old diagnosis was modernized to become more specific in capturing preclinical stages. This, in turn, impressed regulators and is now helping define inclusion criteria for therapeutic trials in predementia AD (e.g., see ARF CTAD story).
In CTE, the biomarker research necessary for similar advances is in its infancy, Stern said. CTE biomarker research can benefit indirectly from natural history studies being done in AD and FTD, but that is not enough. “We need a prospective study, like ADNI or DIAN, for CTE,” said McKee.
Another challenge for academic scientists is that most published research to date has come from athletes. “We want both the diagnostic criteria and the broader research framework to accommodate progressive tauopathy from military trauma, too,” Cummings said.
Mechanistic research needs to tease out how a person goes from an injury to a progressive disease, and why only some people do. Sudden hits to the head rotate and accelerate the brain in a way that is thought to stretch and tear axons, causing what is called diffuse axonal injury (Meythaler et al., 2001; Barkhoudarian et al., 2011). What genetic, environmental, and pathophysiological factors lead from this initial injury to a tauopathy that then spreads relentlessly through the brain? What role do age, inflammation, and glia play in this process? “We need models for that,” said Lee Goldstein of Boston University (see Part 4).
Once a diagnosis, preclinical biomarker trajectories, and a mechanistic rationale are in hand, the goal of preventing CTE in concussed people who show signs of developing progressive disease will be within reach. Tau-based therapies remain slow to come on line, but a few, notably the axonal stabilizer epothilone D, are wending their way through clinical trials. Its current Phase 1b trial in Alzheimer’s was inspired by the research of Kurt Brunden of the University of Pennsylvania, Philadelphia, who spoke at the Lou Ruvo meeting (see ARF related news story).
While researchers focus on mechanisms and drug discovery, advocates such as Nowinski have shifted their attention toward protecting children from sports concussions to prevent future cases (see Part 6 of this series). Both sports and military medicine specialists are developing mobile device applications for coaches and field doctors to better detect concussions where they happen. For example, Jay Alberts of the Department of Biomedical Engineering at the Cleveland Clinic’s home base in Ohio is field-testing a new iPad 2 app in 56 regional high schools. The app measures reaction time, postural stability, and balance errors by the side of the field. It integrates this information with the player’s electronic medical records to render a return-to-play decision during the game. For the military, Hammer mentioned an mTBI Pocket Guide that provides clinical guidance for physicians in theater or far-flung medical stations via their mobile phones. The Surgeon General talked about a National Prevention Strategy instituted by the Obama administration, as well as a central data repository on TBI research which the NIH is building together with the Department of Defense.
The made-for-headlines narrative appeal of youthful star athletes and warriors risking their minds has created an unusual situation. Media attention on CTE has far outpaced the available science. In most areas of science, researchers make progress in relative obscurity before the general media develops an interest. Here, the opposite has happened. The dramatic neuropathology of early cases, football players shooting themselves in the heart to preserve their brains for research, the National Football League’s gradual turnaround, the hardship of veterans struggling to reintegrate at home—all this has fed an appetite for CTE coverage beyond even the nation’s newspapers and ESPN. The television entertainment industry in the past year featured CTE in segments on the TV shows Harry’s Law, Law & Order, The Good Wife, House, and CSI, said Stern.
“We are at such a beginning stage with the science of CTE that it is the outline of the major problems which is now emerging and is allowing us to develop a work agenda. The importance of the conference was to bring the interested parties together so they could identify the low-hanging fruit,” said Cummings.
The Lou Ruvo Center for Brain Health is uniquely positioned to harness the twin powers of celebrity and notoriety for serious research purposes. Las Vegas is the capital of boxing, CTE’s archetypal sport. The star architect Frank Gehry designed the institute’s building with money raised by Keep Memory Alive, a fundraising organization endowed by wealthy philanthropist Larry Ruvo in memory of his father Lou, who suffered from Alzheimer’s. The building is iconic, with a meeting space that flaunts 199 windows, no two of them alike and each fitted with its own motor to shade against the desert sun. The atrium features a 24-foot work by the contemporary American painter James Rosenquist, who reportedly lost family members to Alzheimer’s. Called Brain Space, the painting was commissioned by casino hotel magnate Steve Wynn. The center’s clinic staff trained at the Four Seasons Las Vegas to impart an atmosphere of hospitality—not hospital—when patients and their caregivers come for medical appointments.
Helping to pay off the building and fund the research done inside, Keep Memory Alive hosts events such as its annual Power of Love gala. Last February’s gala celebrated the 70th birthday of Muhammad Ali (see Time U.S. article; Celebrity Net Worth video). The former boxer is said to suffer from parkinsonism, possibly CTE. Stevie Wonder sang "Happy Birthday to Ali" at midnight, President Obama recorded a video greeting, and the night’s 2,000 glamorous guests raised $11 million. Next year’s gala will fete Quincy Jones’ 80th birthday and feature Oprah Winfrey, Cummings said.
The Lou Ruvo Center has started up 27 clinical trials in Alzheimer’s, Parkinson’s, ALS, multiple sclerosis, and Huntington’s, as well as a natural history study of boxers and cage fighters (see Part 2 of this series). “For our patients, we think participating in research is how they can solve their own disease and the disease that afflicts their family. For us, we think science matters more when it generates diagnoses and treatments. It matters less when we only write grants and publish papers,” Cummings said.—Gabrielle Strobel.
This is Part 1 of a six-part series. See also Part 2, Part 3, Part 4, Part 5, Part 6. Read a PDF of the entire series.
Viewed through a scientist’s lens, professional boxing is a series of scheduled, measurable head traumas. In other words, it is a human model for chronic traumatic encephalopathy (CTE). Seen this way, the sport offers a unique opportunity to study the natural history of this proposed progressive tauopathy for an understanding of its presymptomatic pathogenesis and progression. Such data can form the basis for treatment studies and for tools that help boxers decide when to retire from the ring. On 30 September 2012, at a research conference on CTE held at the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, Nevada, Charles Bernick of the Lou Ruvo Center summarized what is known about CTE in boxers and shared early results of his ongoing work with 225 fighters in Las Vegas who have joined a longitudinal biomarker study.
Boxing is the sport that prompted the first phenotypic description of what is now CTE, when the pathologist and Newark medical examiner Harrison Martland coined the term Punch Drunk syndrome (JAMA, 1928;91:1103-1107). The term CTE first showed up in a chapter titled “Punch drunk syndrome: The chronic traumatic encephalopathy of boxers,” that the British neurologist MacDonald Critchley contributed to a 1949 book edited by the French neurosurgeon Clovis Vincent. The current research focus on CTE in the U.S. began with a report on the neuropathology of the former NFL players Terry Long and Mike Webster (see ARF related news story on Omalu et al., 2006).
Epidemiological data on CTE in boxers are sparse, Bernick told the audience in Las Vegas. Some studies put its prevalence among boxers at 17 percent, while others note abnormal brain function in almost all boxers studied. The natural history literature is equally disparate. A predictable course is not yet known. The prevailing estimate is that one-third of cases are progressive, but fighters’ frequent comorbidities such as substance abuse or stroke make that difficult to ascertain. Likewise, some potential risk factors are known—age, number of bouts, number of knockouts, duration of career, ApoE4 status—but those, too, need to be rigorously tested in a natural history study, Bernick said. One particularly fuzzy area is how scientists can measure exposure to cumulative head trauma beyond the bouts and knockouts of known fights. “We do not know what happens in sparring,” Bernick said. “When you ask a fighter if he has ever been knocked out, he’ll say ‘No.’ ‘Have you ever had concussion?’ ‘No.’ ‘Have you ever gotten your bell rung?’ ‘Oh yeah, that happens all the time.’”
In 2011, Bernick started the Professional Fighters Brain Health Study to identify early markers and predictors of CTE. The Nevada Athletic Commission and promoters in town support the study and help spread the word, Bernick said. Ninety-six boxers and 136 mixed martial arts fighters aged 17 to 44—some active, some retired—have joined thus far. Their education level ranges widely from six to 18 years, as does the number of years they have fought professionally (0 to 25) and bouts per year (0-80). “Many are early in their careers,” Bernick said.
The idea is similar to cohort studies such as ADNI. The fighters visit the center for a baseline measurement of family and medical history, education, and genotyping. To estimate exposure, the scientists drew up a composite index that combines data on volume, intensity, and frequency of fights. Participants come in annually for extensive cognitive testing, speech sampling, and to have blood drawn that the study banks for assaying once promising biomarker tests are available. One ELISA claiming to measure all isoforms of tau in serum was recently published (Randall et al., 2012). “This is a highly sensitive assay for tau in serum,” Max Albert Hietala of Gothenburg University in Sweden told the audience in Las Vegas.
The fighters also lie down in the scanner for brain imaging with various MRI modalities including volumetrics, resting-state connectivity, diffusion tensor imaging (DTI), and blood flow. By this point, 45 participants have gone through their second-year tests. In Las Vegas, Bernick summarized the first-year imaging data. On volumetric MRI, the scientists noticed that a variety of brain areas—particularly subcortical ones such as the caudate, putamen, and thalamus—tend to be smaller in fighters who had more exposure. Smaller brain volume was linked to slower processing speed on computerized cognitive testing. Atrophy appeared prior to cognitive findings. Along the same lines, resting-state functional connectivity imaging indicated disconnection in networks involving the caudate and the basal ganglia. On DTI, which images fiber tracts, the corpus callosum appeared to thin out with more exposure. Finally, cerebral blood flow was lowest in people who scored highest on impulsivity tests.
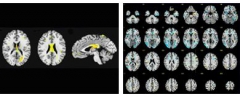
produces these types of brain images, among others: areas of transverse diffusivity indicating fiber tract damage (left) rise the more knockouts a boxer had, and measures of cerebral blood flow (right) decrease with increasing impulsivity. Image courtesy of Charles Bernick, Cleveland Clinic Lou Ruvo Center for Brain Health
These findings broadly recapitulate previous observations in the literature about brain imaging and CTE. Importantly, they come from a single, comprehensively assessed cohort in whom all measures can be compared side by side, rather than as separate bits and pieces from various different convenience samples or retrospective studies, all of which are done slightly differently. “Up to now, radiological evidence has not been terribly helpful in diagnosing CTE. We will need a multimodal approach to distinguish between chronic effects of brain injury and CTE,” said Martha Shenton of Brigham and Women’s Hospital, Boston, who runs the brain imaging component of a separate study of concussed athletes (see Part 5 of this series).
At present, this dataset is still cross sectional. Drawing exposure trajectories from it, Bernick suggested that up to five years of fighting generates few findings, but after that, decline across these measures becomes apparent. “Maybe there is a threshold of what the brain can tolerate,” Bernick said.
Once the study is complete, Bernick hopes to use its data to build a tool that regulatory bodies and boxers can use to assess risk and decide when it is time to stop fighting. As a case in point, Bernick cited International Boxing Federation Welterweight Champion Meldrick “The Kid” Taylor, who continued to fight until 2001, years after he started showing outward evidence of brain injury. It is widely believed that a brutal bout in 1990 in Las Vegas—dubbed Thunder Meets Lightning—damaged Taylor’s brain. A 2003 HBO documentary showed the previously loquacious young man struggling with slurred speech (see more on Taylor).
Does the Cerebrospinal Fluid Flag CTE?
Bernick’s study does not currently collect cerebrospinal fluid CSF samples from its participants. Keeping boxers and martial arts fighters committed to a multiyear intensive study is challenging as it is, Bernick said, though he is considering adding lumbar punctures to the protocol. Extensive previous research from longitudinal cohort studies in Sweden and the U.S. has established an increase in CSF total tau and hyperphosphorylated tau as people develop Alzheimer’s disease. Curiously, the same CSF tau assays are not useful in other tauopathies such as frontotemporal dementia, which shares some symptomatic and brain imaging features with CTE.
Swedish researchers have, however, detected changes in CSF proteins after traumatic brain injury in boxers soon after a bout. In Las Vegas, Hietala told the audience that when a boxer takes a punch to the head, neurons get sheared, axons are injured, and proteins leak out. This is detectable in the CSF. Working with Henrik Zetterberg and Kaj Blennow at Sahlgrenska University Hospital in Gothenburg, Hietala studied 14 Swedish amateur Olympic boxers and 10 healthy controls, with the support of the boxers’ coaches. In CSF samples collected days after a bout, the neurologists detected a sharp increase in the cytoskeleton component neurofilament light protein, plus a less dramatic increase in tau. Both changes receded to normal after three months of rest (Zetterberg et al., 2006). This paper appeared the same year as another one linking CSF tau to a one-year outcome in 39 people with severe brain trauma (Ost et al., 2006). “Neurofilament light protein, in particular, is a robust marker for axonal injury after concussions,” Hietala said.
This clear finding surprised him, because Swedish amateur boxing would be considered “sissy fighting” in the U.S., Hietala said. Wearing extra padded helmets and gloves, boxers face off for only four rounds, and the referee stops the game when a boxer seems groggy.
Either way, boxing is not for "sissies." Image courtesy of Kaj Blennow, Sahlgrenska University Hospital
“This was an explosive topic in Sweden,” Hietala recalled. The medical committee of the Swedish Boxing Association subsequently initiated its own study of 30 amateur Olympic boxers and 25 controls led by Sanna Neselius, a boxer-turned-neurosurgeon. Hietala said it was widely assumed this second study was intended to disprove the initial report. Instead, it resoundingly confirmed it, and even found that in a subgroup of boxers, the CSF elevation of neurofilament light protein and tau did not recover to baseline after several weeks, possibly indicating “ongoing degeneration” (Neselius et al., 2012). Recently, the Swedish researchers have tested the tau assay reported by Randall et al. in samples of these same boxers. “Although the timing of the sampling was optimized for CSF biomarker changes, we saw higher serum tau levels in the boxers,” Zetterberg wrote to Alzforum, adding that future studies may want to take samples hours, not days, after the concussion.
Do as this doctor told you! Sanna Neselius, boxer-turned-neurosurgeon, studies CSF changes in boxers with Max Albert Hietala. Image courtesy of Sanna Neselius
No CSF study on American boxing has yet been published. In 2005, the World Medical Association recommended a ban on boxing.
Not all hits to the head are equally bad, however. In a separate study that measured the same markers in the University of Gothenburg Medical School soccer team, the Swedish neurologists found no changes in the CSF of 23 students who headed 20 corner kicks in a row, even if they got dizzy doing so (Zetterberg et al., 2007). Biomechanics researchers at the Lou Ruvo Center conference noted that when flexed neck muscles support the head in an anticipated soccer header, the uncontrolled, whip-like rotational acceleration of the head that injures axons may not happen.—Gabrielle Strobel.
This is Part 2 of a six-part series. See also Part 1, Part 3, Part 4, Part 5, Part 6. Read a PDF of the entire series.
What exactly goes on in the brains of people when blows to the head they received during sports or military service turn into a degenerative tau disease months or even years after the trauma? The answer to this question is unknown. At a conference on chronic traumatic encephalopathy (CTE) held this fall in Las Vegas, Nevada, a unique session combined human pathology with mouse model data (see Part 4 of this series) to showcase the cutting edge of what is being done to find out. First, Ann McKee of Boston University. “Ann’s research has been transformative. Without the pathologic basis she gave us, we would not know what to think about these cases,” said Jeffrey Cummings of the Cleveland Clinic Lou Ruvo Center for Brain Health, who hosted the conference. “Ann has examined more cases of CTE than any other pathologist in the world,” said Robert Cantu of Boston University.
In 2007, McKee started postmortem studies of concussed athletes and military veterans who donated their brains to the brain bank at BU’s Center for the Study of CTE, or whose families granted permission for autopsy. A review of the CTE pathology literature showed that most described cases up until recently were of boxers (McKee et al., 2009). By now, the majority of the BU samples are football players from high school to the NFL, as well as hockey players and military veterans. Notably, athletes without CTE symptoms have begun pledging their brains to the brain bank in order to provide controls and put the collection on a broader footing.
To date, 136 brains have come into the center’s brain bank, and approximately 100 have been analyzed thus far. About 80 percent had CTE. In Las Vegas, McKee updated the audience on her results. The postmortem pathology of CTE is unique. The gross appearance of the brain changes as the disease advances. Ventricles 2 and 3 expand, and the medial temporal lobe shrinks, as seen in Alzheimer’s. Unlike in AD, the septum pellucidum—a single membrane separating the left and right lateral ventricles—is perforated. Most striking about CTE is the extent and distinctive nature of its tauopathy, McKee said. Hyperphosphorylated tau forms deposits in an irregular, patchy distribution. Curiously, it is densest at the bottom curves of the cortical ribbon, where it engulfs blood vessels. “We see tremendous abnormality at the depth of the sulci,” McKee said, “The sulci are the epicenters of this pathology.”
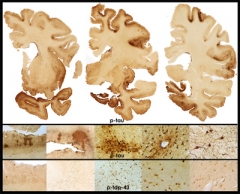
in a 65-year-old former professional football player is evidenced by extensive atrophy and irregular deposits of phospho-tau and TDP-43 proteins throughout frontal and temporal cortices and deep nuclei. Image courtesy of Ann McKee, Boston University School of Medicine/VA Boston.
McKee insists that CTE pathology is distinct from AD. “Most CTE cases I see have no β amyloid, and if they do, then a low amount of diffuse plaques,” McKee said. Earlier studies have reported β amyloid pathology following head trauma (e.g., Roberts et al., 1990; Graham et al., 1995; Roberts et al., 1994). These studies looked early—in some cases only hours or days—in people who had suffered severe injuries, not years after repeated mild injuries. This older work interprets the amyloid deposition as resulting from an acute-phase APP upregulation in response to the trauma. CSF studies have been consistent with that (Olsson et al., 2004). This literature supported the established conclusion that head injury is a risk factor for dementia (for definitive discussion, see AlzRisk meta-analysis).
In McKee’s series, CTE looks like a progressive tauopathy that expands with age. Starting out in frontal cortex, it spreads to insular and temporal cortex, then amygdala and hippocampus. As do frontotemporal dementias and Parkinson’s, CTE also affects subcortical structures such as the thalamus, the hypothalamus, and brainstem, the locus ceruleus and substantia nigra. “There is marked tau deposition throughout the brain,” McKee said. In addition, CTE frequently features TDP-43 deposition, again in a unique distribution, and axonal damage.
McKee is developing a staging system for CTE. As an example for Stage 1, she showed brain slices of a high school football player who died at age 18. “I was very surprised to find tau foci in his frontal cortex,” McKee said. She also showed tau foci in the brain of a 21-year-old team captain in college who hanged himself in his apartment a day after telling his parents he worried about his academics. Stage 1 was apparent in the brain of a U.S. Marine veteran who had shot himself at 28. He had had multiple concussions from a bicycle accident, football practice, combat deployments, and a motor vehicle accident, McKee said. He had had anxiety, as well as trouble concentrating and finding words. Stage 2 features multiple perivascular tauopathy foci and greater evidence of axonal loss. As examples, McKee cited the case of NHL player Derek Boogaard, an enforcer who served 589 penalty minutes in his career. Nicknamed Boogeyman for his toughness, he struggled with depression, and memory and concentration problems, though stays in drug rehab made it difficult to relate causes and symptoms. Boogaard died at 28 (see NYT video series). Similar CTE pathology occurred in the brain of a 45-year-old Operation Enduring Freedom/Operation Iraqi Freedom army veteran who had been exposed to a single close-range IED blast, McKee told the audience in Las Vegas.
As an example of Stage 3, which is marked by further spread of tau deposits and axonopathy, McKee showed the case of Dave Duerson, who shot himself in the chest after leaving a note requesting that his family donate his brain to research. Stage 4 represents endstage CTE. McKee showed the brain of an 80-year-old Football Hall of Famer who was also an army veteran. His septum was broken and the brain shrunken, the medial-temporal lobe a third of its normal age-controlled weight. “Even he had no β amyloid, but extreme tauopathy everywhere in the brain,” McKee said. At each stage, some athletes and blast-exposed veterans appeared indistinguishable pathologically.
Like most neurodegenerative diseases, CTE comes in variants. Of the current set of 68 brains, 51 represent pure CTE, but eight were a combination of CTE and motor neuron disease. The other nine included multiple pathologies that met diagnostic criteria for AD, Parkinson’s/dementia with Lewy bodies (DLB), or frontotemporal dementia (FTD). The ALS-like disorder has drawn particular attention (see ARF related news story). McKee cited the case of a college football player who had started the sport at age eight and had sustained many concussions. He contacted McKee and Chris Nowinski at Boston University, who co-founded the Center for the Study of CTE, and shortly before he died from ALS-like symptoms, asked that his brain be studied. It showed profound CTE and TDP-43 proteinopathy in the spinal cord.
This pathology suggests that CTE and its variants may underlie some clinical diagnoses of AD, PD, and ALS given to former athletes and soldiers who display symptoms of these better-known diseases, said Cantu. At the Lou Ruvo conference, Cantu cited a widely noted study that reported initial epidemiological numbers for the incidence of Alzheimer’s, Parkinson’s, and ALS in former NFL players (see ARF related news story). This study represents the best incidence numbers so far, but even so, it may be skewed in that some of those diagnoses, made without autopsy confirmation, probably represent CTE and its variants, Cantu said. The study analyzed death certificates, not the brains of the players. As such, it is also subject to undercounting, as causes of death are often incompletely noted on these forms. “I have filled out many death certificates over the years. In the distress of the situation, doctors are often less detailed than they could be,” Cantu said, “We still do not know the incidence and prevalence of CTE. Death certificates are a start, but we need to study brains and use autopsy-confirmed diagnoses.”—Gabrielle Strobel.
This is Part 3 of a six-part series. See also Part 1, Part 2, Part 4, Part 5, Part 6. Read a PDF of the entire series.
No Available Comments
A growing number of researchers view age-related neurodegenerative diseases as being due to pathogenic alter egos of otherwise normal proteins. The idea is that the alter egos self-propagate from cell to cell in a steady march through the brain along its anatomic or functional networks. The proteins vary—Aβ and tau in Alzheimer’s, α-synuclein in Parkinson’s, huntingtin in Huntington’s. The driving forces vary—genetic in Huntington’s, genetic or sporadic in Alzheimer’s and Parkinson’s, infectious in rare human cases of variant Creutzfeldt-Jakob disease. At the Chronic Traumatic Encephalopathy conference held 30 September to 1 October 2012 at the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, Nevada, Stanley Prusiner of University of California, San Francisco, presented data suggesting that brain trauma, too, can cause such a spread of a pathogenic protein—in this case, tau.
Prusiner in 1997 won the Nobel Prize in Physiology or Medicine for his group’s research on prions as a new infectious principle. In the 1990s, a separate line of research into potential self-propagation of amyloid-β began when British scientists reported that injecting brain extracts of AD or Down’s syndrome brains into marmoset brains accelerated the spread of this pathology far beyond the injection site into remote areas of the marmosets' brains (e.g., Baker et al., 1994). This research became prominent in the AD field when Lary Walker and Mathias Jucker adapted and expanded the experimental paradigm in AD mouse models, and soon after Eliezer Masliah’s, Mark Diamond’s, and others' groups showed similar spreading after a single inoculation of α-synuclein and tau. Using anatomical staging, Heiko Braak’s research suggested the spread of disease proteins for Parkinson’s and AD. The movement of the propagating proteins is a slow process. That explains why these diseases, even the most aggressive autosomal-dominant forms, do not express themselves at birth but take decades to develop, Prusiner said.
At the Lou Ruvo Center conference, Prusiner claimed that CTE might be the newest member of these diseases, adding post-traumatic changes in the brain as a new cause. In his talk, Prusiner focused on FTD and CTE as diseases that occupy a new interface between psychiatry and neurology. While those two diseases are distinct, they do share some symptoms. And, according to Prusiner, both are likely to be caused by the self-propagation of tau prions.
Prusiner showed new data generated with an established experimental system using bigenic mice. These mice not only express the P301S FTDP-17 mutation, but also conveniently display tau deposition in the brain with a rise in bioluminescence that can be quantified over time without having to sacrifice the mice for pathology studies (see Tamgüney et al., 2009; Watts et al., 2011). The mice show a spontaneous rise in bioluminescence at 160 to 170 days of age. If the scientists inject extract from cognitively normal 80-year-old people as controls, that number stays the same. However, it was strikingly different when the scientists injected extract from three different human brains into these mice. One was from a patient who died from progressive supranuclear palsy, a pure tauopathy, and one from a person who died with Pick’s disease, the quintessential tauopathy; both were donated by Bruce Miller’s group at the University of California, San Francisco. Finally, the scientists injected extract from an 80-year-old football player with stage 4 CTE donated by Ann McKee at Boston University. Each of these three samples led to a sustained rise in bioluminescence starting between 83 and 91 days. Subsequent histochemistry confirmed widespread tau deposition at that age, Prusiner said. “At 60 days after inoculation, we see the rise in bioluminescence,” Prusiner told the audience.
“Prion-like behavior of the tau protein could turn out to be the way to address progressive degeneration after the end of the trauma,” said Jeffrey Cummings of the Lou Ruvo Center. How does tau move from cell to cell? For his part, Prusiner suggested that aggregating tau inside the neurons represents the pathogenic agent. He believes it polymerizes and travels to the synapse, where it leaves the cell and enters another. McKee noted that CTE pathology suggests that there is significant extracellular spread as well, quite possibly via glial cells.
Other leaders of neurodegenerative research are advising against the use of the word “prion” in the context of Alzheimer’s, Parkinson’s, Huntington’s, and CTE, for that matter, because none of these diseases spreads from animals to animals, animals to humans, or humans to humans. The spread is from cell to cell, strictly within one organism. To avoid the infectious disease connotation of the term “prion,” AD researchers increasingly refer to this phenomenon as "templating" (e.g., Hardy and Revesz, 2012; ARF related news story).
Research with mice is important not only to understand how CTE develops, but also to build better models for therapy development, Prusiner said. Because the bigenic bioluminescence model predictably turns on deposition two months after inoculation, he considers it suitable for evaluating candidate drugs for the disease.
Likewise, Lee Goldstein of Boston University pronounced his group’s mouse model of CTE validated for drug testing. This model is different. It does not model the hypothesized mode of protein propagation as much as reproduce the initial head trauma people suffer in order to recapitulate the cellular and functional phenotype that unravels from there. To Goldstein, tau’s predilection to deposit in the cortical sulcus carried a "whispering of physics," he said in Las Vegas. He teamed up with blast experts to build a shock tube. It delivers to wild-type mice a pressure wave, immediately followed by a blast wind of more than 350 miles per hour, similar to what a soldier would experience from an IED. A previous ARF related news story covered the published findings on this model. At the Lou Ruvo Center conference, Goldstein noted that, since then, he was struck to see the mice reproduce a feature of human CTE he considers important, if not understood. “The pathology engulfing the vasculature in the affected regions is massive, yet right nearby are blood vessels that are totally normal,” Goldstein said.
After the blast itself, the brains of these mice look normal at a macroscopic level. There is no blood, no crush, no rupture injury. But already two weeks after a single blast, the scientists see a variety of hyperphosphorylated tau isoforms and tauopathy. At that point, Goldstein said, whole brain areas appear wiped clean of living cells. In particular, electron microscopy visualizes extraordinary changes to the cytoarchitecture of the blood-brain barrier. “Astrocytic end feet are filled with fluid. They look very sick. The damage to the barrier is pervasive,” Goldstein said.
Further study has shown evidence of extravasation in both directions, compromising the functional integrity of the barrier. “I would not be surprised if we got invasion of T cells or other peripheral cells into the brain,” Goldstein said. For this reason, the BU group currently focuses on detecting CNS biomarkers in the periphery. Previous research has already detected tau and neurofilament light chain as markers of axonopathy in the CSF (see Part 2 of this series); a blood assay for tau was recently published (Randall et al. 2012).
The cellular damage appears to have functional consequences. Their axonal conduction is slow and LTP disrupted. In the Barnes maze, a hippocampal learning paradigm in which the mouse learns to find a dark hole that allows it to escape from a lit, exposed table, blast-exposed mice cannot remember where the hole is.
“Our animal model is ready for drug testing. It is validated for phospho-tauopathy, for diffuse axonopathy, and many other characteristics of CTE. It replicates what we see in humans who are injured on the ball field and in battlefield,” Goldstein said. In other neurodegenerative diseases, researchers are recommending that in-depth characterization of mouse models and subsequent quality controls with large group numbers precede preclinical drug studies (see ARF related news story).—Gabrielle Strobel.
This is Part 4 of a six-part series. See also Part 1, Part 2, Part 3, Part 5, Part 6. Read a PDF of the entire series.
No Available Comments
When a new disease emerges, clinicians grapple early on with how to diagnose it. This is true for chronic traumatic encephalopathy (CTE), the degenerative tauopathy that develops in some people after they receive a blow to the head in the course of contact sports or military service. Researchers do not know how common the disease is. They do not have biomarkers for it. There are no treatment trials for it. “At the very base of asking any of these questions is, Who is a case? That is why we need to advance consensus diagnostic criteria,” said Jeffrey Cummings, who hosted a recent conference on CTE at the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, Nevada.
At this meeting, Robert Stern of Boston University for the first time presented a draft version of research-based criteria to any kind of public forum. The time has come to focus the field’s attention on consensus criteria, because the pathology of CTE by now is fairly clear and physicians are beginning to diagnose the condition during a patient’s lifetime, but they are doing so each in their own way. CTE at present is tricky to diagnose, Stern said. “We must differentiate it from Alzheimer’s, from frontotemporal dementia, and from chronic stress. We need to diagnose and then follow people with CTE, both to study progression and to try to protect them from suicide, which is a big problem in this population.” Moreover, purely clinical, phenotypic diagnoses are often inaccurate; objective tests should be built into it.
Stern recounted how recent progress in the Alzheimer’s field has inspired his group’s attempt to get off the starting blocks. In AD, criteria dating back to 1984 used clinical and paper-and-pencil psychometric tests to diagnose dementia. A definitive diagnosis had to await autopsy, much like CTE today. In AD, this has given way to a much more accurate lifetime diagnosis. The formal change began five years ago with a biomarker-driven diagnosis for research purposes (Dubois et al., 2007), which in turn spurred the development of new diagnostic criteria for earlier-stage disease by other leading groups as well (see Alzforum Webinar). There has been debate about the attendant terminology in this shift, and different groups have accepted biomarkers to a differing degree, but in essence, Alzheimer’s disease has moved to being diagnosed during a person’s lifetime at early stages, before the person shows the overt dementia that is increasingly viewed as the disease’s endstage. Years of convergent findings on brain imaging and cerebrospinal fluid protein measurements from natural history cohorts in Europe, the U.S., Australia, and Japan now anchor this change. Knowing the trajectory of biomarker change in the years before people develop symptoms is transforming clinical trials in AD toward preclinical treatment that aims to delay the clinical presentation (see CTAD 2012 Alzforum series). By learning from AD, CTE research can advance toward similar trials more quickly, Stern said.
To create diagnostic criteria for CTE, Stern and colleagues first reviewed the world literature, then interviewed the families and studied the medical records of the 68 CTE cases Ann McKee has analyzed recently (see Part 3 of this series). They used information on the 40 of these 68 who had "pure" CTE without motor neuron disease or other major confounds, and whose last brain trauma dated back at least five years to eliminate the immediate effects of concussion. These 40 had died at ages 22 to 98 at CTE stages 1 through 4 of suicide, drug overdose, dementia, or other medical conditions. The primary symptoms loved ones reported fell into four groups. Cognitive problems centered on memory, executive function, and dementia, as in AD. Behavioral problems included a short fuse, aggression/violence, and substance abuse, not the inappropriate touching and public behavior that is typical of frontotemporal dementia. Mood problems included apathy—a known symptom of certain FTDs—but also sadness, suicidality, hopelessness, and irritability. Motor disturbances included falls, disturbed gait, poor balance, and parkinsonism. Behavioral and motor symptoms tend to precede cognitive ones, and the number of symptoms grew as people advanced to stage 4.
Candidate biomarkers for CTE exist. Besides elevated CSF tau with normal CSF Aβ, they include a handful of MRI modalities, a biochemical metabolite signature by magnetic resonance spectroscopy (MRS), and amyloid PET to rule out AD. Tau PET ligands for CTE are much anticipated. EEG as measured by BrainScope, a handheld tool used by the military, has also shown promise. However, none is fully studied for inclusion in a CTE consensus diagnosis.
For a start, Stern leads a cohort study called Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests, aka DETECT. This interdisciplinary study is the first CTE program funded by the National Institutes of Health. It measures side by side a range of biomarkers in 100 symptomatic former NFL players who played positions that tend to take severe hits to the head most often, and compares their data to those of 50 former Olympians in sports not prone to concussions. All participants come to Boston for two packed days of assessments, clinical and neuropsychological tests, and a blood draw to analyze genetic risk factors. “We have been very successful in recruitment and expect to get this done quickly,” Stern said. The study started in the fall of 2011 and is a collaboration between BU, Les Shaw at the University of Pennsylvania for CSF analysis, outside academic consultants on head trauma and suicide, and Brigham and Women’s Hospital in Boston for brain imaging.
In Las Vegas, Martha Shenton of Brigham and Women’s, who leads DETECT's imaging component, noted that a pilot study on five concussed former athletes confirmed some of the findings reported in the literature, seen by Ann McKee (see Part 3), and also seen in Charles Bernick’s study of boxers and cage fighters (see Part 2). These include holes in the septum pellucidum membrane, a shrunken cortex, a thinned corpus callosum, and fiber damage revealed by a new imaging modality called tractography.
What about milder hits to the head? Do they cause changes that brain imaging can pick up? In the November 13 Journal of the American Medical Association, Shenton published results of a DTI study comparing the integrity of the white matter in 12 elite German soccer players, whose frequent heading exposes them to sub-concussive blows, to that in 11 elite swimmers. Working with colleagues at the Ludwig-Maximilians-Universität in Munich, Germany, Shenton’s group found greater radial diffusivity in many brain areas, and greater axial diffusivity in the corpus callosum. Only visible with advanced DTI—not on regular MRI—these kinds of subtle changes do, however, suggest possible demyelination, the authors write (Koerte et al., 2012).
Back to DETECT. Even if this larger study finds clear differences in the biomarker signature between the full cohort of football players and the controls, they will not constitute proof that those changes are due to CTE. “From a cross-sectional measurement, we cannot distinguish if we are seeing changes from the repetitive brain trauma that might be chronic, or evidence of a new, progressive disease,” Stern said. For that, a prospective natural history study that yields progression slopes will be necessary.
With current knowledge in mind, Stern took a first stab at research diagnostic criteria. They stipulate a minimum age, a history of brain trauma exposure, and definition of the identity and progressive nature of symptoms. Borrowing from Alzheimer’s, Stern proposes five diagnostic categories—preclinical CTE, possible early-stage CTE, probable early-stage CTE, possible CTE dementia, and probable CTE dementia—and lists biomarker and symptom combinations for each. This first draft is currently incorporating feedback from other CTE researchers, and will then be published, Stern said. At that point, the CTE diagnosis may be close to where the Alzheimer’s field was in 2007, when biomarker research criteria were published and awaiting validation. “Our criteria are a ways off from being ready for clinical use,” Stern said.
Before that happens, the draft’s framework will also need to expand to include military trauma so that soldiers can be diagnosed with the final diagnostic criteria as well, Cummings said. Most of the work to date has come from athletes.—Gabrielle Strobel.
This is Part 5 of a six-part series. See also Part 1, Part 2, Part 3, Part 4, Part 6. Read a PDF of the entire series.
No Available Comments
When a Pittsburgh medical examiner reported chronic traumatic encephalopathy in former Steelers players who had died young (Omalu et al., 2005; Omalu et al., 2006), the specter of neurodegeneration in concussed athletes quickly gained momentum. Close to the New England Patriots' hometown, Christopher Nowinski, a former Harvard football player and cage wrestler, joined forces with concussion expert Robert Cantu to found the advocacy organization Sports Legacy Institute in 2007. In 2008 they partnered with neuropathologist Ann McKee and clinical neurologist Robert Stern to form the Center for the Study of Traumatic Encephalopathy at Boston University School of Medicine. Together, the group published a review of known cases to date, putting CTE on the map as a progressive tauopathy and starting a national media drive on the topic (McKee et al., 2009). Last month in Las Vegas, Nevada, these scientists, Nowinski, and many others gathered for a conference on CTE at the Cleveland Clinic Lou Ruvo Center for Brain Health to articulate a research agenda for CTE (see Part 1, Part 2, Part 3, Part 4, and Part 5 of this series). The advocacy of the past five years has forced some changes. It has recently opened a second front by fighting concussions in contact sports in children.
The film Head Games documents the dangers of head trauma, particularly to children who play contact sports. (Click on image to view film trailer.)
The NFL stonewalled for a while, Cantu told the audience. Famously, Ira Casson, formerly of the league’s TBI committee, earned the moniker “Dr. No” for his one-word answers in an HBO interview to questions about links between multiple head injuries and neurologic consequences including dementia. Casson also avoided clear statements in a 2008 Alzforum Live Discussion on the topic with leading CTE researchers (see transcript).
Recently, however, the NFL made changes to reduce head trauma. It moved the kickoff line, banned concussion-prone maneuvers such as the three- or four-man wedge, and forbade hitting an unseeing opponent above the neck. When players lose consciousness or are symptomatic, they have to stay out of the game or the practice that day. Amateur leagues followed these NFL reforms within months, Cantu said. Moreover, NFL teams must now have an independent neurologist who decides when it is safe for the athlete to return to play. The league imposed penalties up to suspension for hitting the head of a "defenseless" player, that is, someone looking at the ball. A referee can now send a player suspected of having a concussion to the sideline to be checked by the medical team, as is done in martial arts. Implementation continues to be marked by conflict, however (e.g., see ESPN story).
Importantly, said Nowinski, NFL games now ensconce an athletic trainer high up in a skybox overlooking the field who views instant TV replays and can call players suspected of a concussion to come to the sideline for a medical check. “These guys up high see more concussions than sideline refs,” said Nowinski. The changes are not enough, Cantu said. “If the NFL wants to go to the next level in player protection, it needs to make all intentional hits to the head illegal.”
The NFL is directing funding to the problem. In September, timed to the start of the regular season, the league announced a $30 million donation to the Foundation of the National Institutes of Health in its single largest grant to any organization in the past, according to the league. Part of the NFL’s agreement with the players' union, this gift is meant for research on brain injuries that will benefit soldiers as well as athletes and the general population. NFL Charities, the foundation of NFL owners, is providing $1.5 million for 15 research grants, such as a recent one to the Concussion Management Clinic at the University of Buffalo School of Medicine, to develop scientific methods to determine when an athlete can safely return to play.
On Thursday 15 November, NFL commissioner Roger Goodell addressed the Harvard School of Public Health after three quarterbacks had sustained concussions in the previous Sunday’s games, and on 19 November, Goodell announced that the league would convert players’ medical records to an electronic health record system (see Goodell video/story).
For their part, Nowinski and Cantu have shifted their attention to protecting children. “We know that diagnosis and treatment development will take years,” Nowinski said. “Meanwhile, we can prevent future cases now.”
At the Las Vegas conference, Nowinski reminded researchers that children are at higher risk of CTE than adults because the axons in their central nervous systems have less protective myelination. Myelination continues throughout a person’s youth. Their heads are relatively larger than those of adults compared to their body size, and their neck muscles are weaker. “Essentially, they are like bobble head dolls when it comes to being whacked on the head. They are at a biomechanical disadvantage,” Nowinski said. Helmet sensors such as built-in accelerometers and gyroscopes are telling scientists that the force of a hit to the head for a high school football player is about the same as the main force to the head for college players, about 18 to 22 gravity force (G force). “For young kids it is 15 G force. That is much too high for a large head on a weak neck,” said Nowinski.
Blaine Hoshizaki, of the School of Human Kinetics at Ottawa University, studies the head’s dynamic response to different types of hits—falls, collisions, punches, and impact from flying objects such as pucks and baseballs. Hoshizaki’s team reconstructs real head injuries captured on video, for example, of this NHL hit that kept the concussed player off the ice for a year. The Ottawa scientists measure the dynamic response of the head, including both linear and angular head acceleration in the x-, y-, and z-axes, and use those data to calculate brain tissue deformation that follows from it. Present-day helmet standards only employ linear acceleration to measure helmet protection, while it is angular acceleration that is more closely associated with concussive injuries. It is important to develop a testing protocol for evaluating helmets that include both linear and angular acceleration, Hoshizaki told the Las Vegas conference audience. He also said that the type of impact to the head determines the resulting trauma to the brain. Depending on whether the athlete was part of a collision, fell to the ground, was punched, or hit by an object determines the location and type of brain tissue damage.
The Sports Legacy Institute is now pushing a hit count initiative. The idea follows the more established pitch counts that protect the elbows of young baseball players, with national limits to how many pitches a youngster can throw and mandated rest (see White Paper). “We have nothing similar for hits to the head yet. Hit count data in the literature are as high as several thousand in high school, more than half of it in practice,” said Nowinski.
While middle and high schools have started baseline concussion testing for most field sports at the beginning of each academic year, youth football in particular continues to proceed without medical training requirements or revised practice procedures for coaches. The issue is gaining attention in the media, rattling parents and Pop Warner itself. On 23 October, The New York Times ran a story on a pee-wee game in central Massachusetts that carried on to a final score of 52-0 even as five 10-year-olds received head injuries. That evening, National Public Radio quoted Cantu as saying that boys younger than 14 should not play collision sports, a position he also took at the Las Vegas conference. Cantu recently published a book on the subject (Concussions and Our Kids). This fall, director Steve James, of Hoop Dreams fame, released his new documentary film Head Games, based on Nowinski’s book of the same title.
“Is this the beginning of the end of football? No. But maybe for young kids it is,” Nowinski said.—Gabrielle Strobel.
This concludes a six-part series. See Part 1, Part 2, Part 3, Part 4, Part 5. Read a PDF of the entire series.
No Available Comments
Comments
No Available Comments
Make a Comment
To make a comment you must login or register.