CONFERENCE COVERAGE SERIES
Human Amyloid Imaging 2012
Miami, FL, U.S.A.
12 – 13 January 2012
CONFERENCE COVERAGE SERIES
Miami, FL, U.S.A.
12 – 13 January 2012
When 225 researchers from around the world convened in Miami to exchange the latest on β amyloid positron emission tomography (PET) on 12-13 January 2012, there was a palpable sense of urgency. At the 6th Annual Human Amyloid Imaging (HAI) Conference, the specter of potential approval, possibly within months, of the first radioligand for clinical use hung over the room. Hence, as scientists openly discussed new developments on all fronts of this young field, several themes stood out for reaching beyond the research data themselves. Scientists wrestled with whether and how to disclose amyloid status to people without dementia. They discussed how they would use amyloid PET in the clinic. They focused on methods to read whether a person’s scan is positive just by looking at it versus more quantitative ways of interpreting it.
On the research front, there was ample news to share as more groups enter the field and data accrue. Perhaps most importantly, some 150 presymptomatic carriers of Alzheimer’s disease (AD) mutations in their twenties, thirties, and forties by now have had amyloid scans. Researchers are getting a stronger sense of how ApoE and age influence amyloid deposition, and are starting to try to pinpoint when and where this pathology first crops up in the brain. Right up there in researchers' estimation were the growing number of longitudinal studies that follow cognitively normal and mildly impaired people at varying degrees of genetic risk. The overall trend of those studies appears to be that brain amyloid deposition is bad news, though it can take years until a given person suffers cognitive consequences, and the initial cognitive decrements are subtle. “I think the notion that amyloid has no effect in people who are asymptomatic is not supported by data,” said Denise Park of the University of Texas at Dallas, whose group published a paper to this effect (Rodrigue et al., 2012).
But even as longitudinal studies converge, all does not fit. The conference brought to a boil a theme that has been simmering: In a significant fraction of cases, amyloid imaging disagrees with a patient’s clinical diagnosis, and more research is needed to find out which of the two is correct. This process may shake up the diagnosis of AD and, indeed, numerous related forms of dementia. It may give unexpected prominence to non-AD dementias, for example, the frontotemporal varieties. To put diagnosis of AD and FTD on more of a molecular imaging plane, the field urgently needs ligands to trace all defining pathologies, above all, neurofibrillary tangles. At the HAI meeting, the first tau-only PET data in humans received rapt attention; two more compounds are in late preclinical stages. (For nuclear medicine to delineate AD and dementia with Lewy bodies, an α-synuclein tracer is needed, but none appears in sight yet.) This news series will cover each of these points in a dedicated story below.
Co-organized by Keith Johnson of Massachusetts General Hospital, with help from Bill Klunk and Chet Mathis at the University of Pittsburgh Medical School, Pennsylvania, and Bill Jagust of the University of California, Berkeley, the HAI Conference has doubled in size from its beginnings in Boston in 2005. It stands out among conferences in that the organizers assemble the program from among the submitted abstracts only a month prior to the actual meeting, and budget ample discussion time into every session and between sessions—both in an attempt to offer a forum in which new data can be shared and absorbed in depth. The conference recognizes junior investigators in the field with a Young Investigator Award. This year it went to Manja Lehmann of the University of California, San Francisco, for her multimodal imaging study linking atrophy and hypometabolism by FDG-PET in functional networks of the brain that are specifically affected in the language or visual variant of AD as compared to its predominant amnestic presentation. Lehmann found that this signature identified each variant, while amyloid PET was positive more globally in all three variants of AD and did not distinguish among them. This, Lehmann told the audience, would imply that amyloid imaging can flag AD but not explain its heterogeneity, as factors other than amyloid explain region-specific patterns of neurodegeneration and atrophy in the disease’s subtypes.
HAI encourages young scientists by awarding travel scholarships. These went to Rebecca Amariglio of Brigham & Women's Hospital, Boston (Amariglio et al., 2012), Gerard Bischof of the University of Texas at Dallas (Bischof et al., 2012), Amarallys Cintron of Emory University, Atlanta, Georgia (Cintron et al., 2012), Manja Lehmann of the University of California, San Francisco (Lehmann et al., 2012), Natalie Marchant of the University of California, Berkeley (Marchant et al., 2012), Hwamee Oh of the University of California, Berkeley (Oh et al., 2012), Ozioma Okonkwo of the University of Wisconsin, Madison (Okonkwo et al., 2012), Rik Ossenkoppele of VU University Medical Center, Leuven, Belgium (Ossenkoppele et al., 2012), Jenny Rieck of the University of Texas at Dallas (Rieck et al., 2012), Bedda Rosario of the University of Pittsburgh, Pennsylvania (Rosario et al., 2012), Pascual Sanchez-Juan of University Hospital Marqués de Valdecilla (Sanchez-Juan et al., 2012), and Miranka Wirth of the University of California, Berkeley (Wirth et al., 2012).

Comparison between AD patients and controls for FDG-PET and PIB-PET. (View larger image.) FDG patterns correspond to clinical phenotype: largely temporoparietal hypometabolism in typical AD, left hemisphere predominant in language-predominant AD, and posterior predominant in visual AD. PIB patterns are diffuse and generalized in all clinical groups. Image credit: Manja Lehmann, Bill Jagust, UC Berkeley
The organizers anchor presentations on detailed imaging and related biomarker data with a technical lecture aimed at helping the field improve techniques and eventually arrive at best practices. In his lecture, Robert Koeppe of the University of Michigan at Ann Arbor offered the advice of a widely regarded leader in PET to his colleagues in the trenches of amyloid imaging. He reassured the field that the target regions for an amyloid scan indicating AD pathology are well established and replicate well across studies. However, Koeppe said, studies pinpointing when and where amyloid deposition begins are well advised to use whole brain scans. One open question with which researchers wrestle is what reference region is best. Many use the cerebellum because it contains little amyloid in LOAD, but it is not ideal for longitudinal research or studies of familial AD. Pons or even white matter are being studied as alternatives, and Koeppe said that quantitative research would do best to combine all three and normalize against that. On a seemingly arcane technical point that last year pervaded discussions at HAI—whether to try to correct for atrophy and how best to do so—Koeppe advised scientists to keep things simple. Partial volume correction adds enough uncertainty and error, Koeppe said, that it is generally not worth the trouble, except in longitudinal studies (Koeppe, 2012).
A question that reliably buzzes in hallway chats among nuclear medicine physicians is how the present crop of 18F compounds, notably florbetapir, the furthest advanced, compare to Pittsburgh compound B (11C-PIB). Side-by-side comparisons of radioligands are rare, but Koeppe found one. He analyzed data on 29 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) who underwent first a PIB and then a florbetapir scan on the same scanner. Koeppe said the main difference between the compounds lies not, as is often thought, in florbetapir’s slightly higher white matter binding. Rather, he said, it is florbetapir’s lower cortical signal, which in turn is partly offset by its lower noise. In essence, both tracers worked for visual reads of clearly positive or clearly negative scans, Koeppe said, though for scans on the borderline, the florbetapir signal being about 60 percent that of the PIB signal may become significant. This would not interfere with routine diagnostic use but might be a disadvantage when trying to quantify precisely the first appearance of amyloid deposition, small longitudinal changes, or small drug effects, Koeppe said, and other scientists at HAI largely agreed. Researchers from companies that are beginning to incorporate amyloid PET into their treatment trials said they consider these differences minor.
HAI shakes up its all-imaging-all-the-time diet with two lectures from related fields in AD research. In the first of this year’s keynote lectures, Vahram Haroutunian of Mt. Sinai School of Medicine in New York City described how studying age-related changes in the brain’s transcriptome based on autopsy samples suggests that scientists broaden their view of dementia beyond the boundaries of plaques and tangles. This work suggests that in order to escape dementia, the aging brain somehow needs to retain its ability to respond robustly to factors that are causing dementia, from Aβ to tau to α-synuclein and other insults. This notably requires transcription of protective immune and anti-inflammatory genes, Haroutunian said (Haroutunian, 2012). Claudia Kawas of the University of California, Irvine, followed with a keynote on the 90+ Study, a longitudinal, community-based prospective observation of representatives of the fastest-growing segment of the population. In a nutshell, this nine-year-old study of some 900 people in their nineties has shown that the prevalence of dementia in the oldest old doubles every five years, just as it does in people’s seventies and eighties, dispelling the common perception that the very old have somehow escaped dementia. The 90+ Study further showed that the suite of proposed AD risk factors—from vitamins, alcohol intake, caffeine, homocysteine, and others—does not protect against dementia in very old age. In contrast, and tantalizing to the assembled HAI audience, autopsy analysis from the 90+ Study showed that the total area of brain amyloid and tau pathology correlated with people’s cognitive decline before they died (Robinson et al., 2011). Overall plaque area was more strongly related than any particular type of plaque. And more recently, initial amyloid imaging results in a small subgroup of the 90+ Study participants appear to suggest that, even among the oldest old, people decline rapidly in the 1.5 years after having had a positive scan, Kawas said (Kawas, 2012).
If the finding on plaque area and cognition holds true, it might simplify things for amyloid imaging, said Rik Vandenberghe of Catholic University, Leuven, Belgium. It might put aside ongoing debate about which plaque to call what and how strongly each type binds PET tracers. Plaque area is a continuous measure that is more straightforward in practice than having to distinguish between types of plaques, Vandenberghe added.
The program book containing all abstracts, as well as video recordings of two keynote lectures, has been posted and is freely available at the HAI website.—Gabrielle Strobel.
This is Part 1 of a nine-part series. See also Part 2, Part 3, Part 4, , Part 5, Part 6, Part 7, Part 8, Part 9. Download a PDF of the entire series.
The first amyloid imaging ligand that used fluorine 18, florbetapir F18 (Amyvid™), is under regulatory review in the U.S. and Europe. A company spokeswoman confirmed that she expects the Food and Drug Administration to act in the first half of 2012. At the 6th Human Amyloid Imaging Conference, held 12-13 January 2012 in Miami, Florida, researchers who had gathered primarily to share breaking research news all knew this, and spent time anticipating a world where amyloid PET is entering clinical practice beyond its current narrow use in research studies and therapeutic trials. Scientists discussed how this could be done in the best interest of people who do not have dementia but may be on the way. This story distills the main points of view expressed during a conference session that set aside formal data presentation to instead host an airing of the issues to come once amyloid imaging becomes more widely available.
This conference drew primarily scientists in academia, industry, and government, as well as some physicians in private practice. Many of the academic researchers have potential conflicts of interest with companies making tracers, scanners, or AD drugs. They agreed that amyloid imaging will be useful to identify people pre-dementia who are candidates for inclusion into anti-amyloid treatment trials. This was seen as true even though work remains to be done to tighten researchers' ability to predict when an amyloid-positive person will develop symptoms and which second and third hits—ranging from cerebrovascular disease to cognitive reserve to other factors—determine the rate of this progression. Screening for trials, however, is still a research use. The real open question to many clinicians at the conference was: How will amyloid imaging occur in routine clinical care?
Chris Rowe of the University of Melbourne, Australia, began the discussion, asking first, “Why do we want amyloid imaging?” For one thing, he said, because people want to know. At least in Rowe’s catchment area, community demand for diagnostic and prognostic information is strong. Studies have shown that people cope better when they have information and early diagnosis so they can plan. For another, it is often useful in diagnosis, Rowe said. Particularly for people with MCI, predictions of progression to AD are emerging from ongoing longitudinal studies with “very high consistency.”
Rowe foresees using amyloid imaging when he believes it will affect the management of a given patient’s disease. This means he would not use it for most patients with typical AD. Cognitive tests plus an MRI with automated hippocampal volume measurement are accurate for a typical 75-year-old patient with a medical history and clear dementia. However, if a patient is 60 and still employed, Rowe would use an amyloid scan. Ditto for a patient with suspected FTLD that looks as if it might be AD instead. In FTLD cases, amyloid PET has had no false-positive scans against postmortem pathology; hence, using it to clarify a patient’s diagnosis is reliable and would affect whether that person is offered AD drugs, Rowe said. Other examples where amyloid imaging could help in day-to-day diagnosis include distinguishing semantic dementia and vascular dementia from AD where, again, only the latter would be offered a prescription of the currently approved AD drugs. In subsequent discussion, there was broad agreement that amyloid PET can help confirm a non-AD dementia diagnosis.
Rowe advised great caution when determining whether a patient’s apparent dementia might be due to depression or early AD. Both amyloid deposition and depression are sufficiently common that it is possible amyloid might occur coincidentally in a person with severe depression. If overinterpreted, an amyloid scan in a depressed, cognitively impaired person could prompt a false diagnosis of AD. In these cases, an FDG-PET can help sort out the cause of the person’s suffering.
In summary, in MCI cases, Rowe would use amyloid PET to determine if patients are suffering from prodromal AD or if their impairment is due to other causes. In people with subjective memory complaints, Rowe would use amyloid PET if cognitive tests indeed showed abnormal findings. In cognitively normal people, Rowe would use amyloid imaging only to recruit for treatment trials. In all, a significant fraction of cases are uncertain enough to warrant amyloid imaging, Rowe said.
“Imaging has produced quite an armamentarium to help diagnose AD,” said Bill Jagust of the University of California, Berkeley. “This is an extremely expensive workup for a disease that has no good drugs. Will that happen?” The answer depends on who will pay for amyloid imaging. At HAI, some physicians predicted that it would only be widely used if government or private insurance covers it, whereas physicians from other areas, such as the host city of Miami, expected significant use for a one-time scan by people who pay out of pocket. In the U.S., the rollout of amyloid imaging could become a test case for the Affordable Care Act, said Jason Karlawish, of the University of Pennsylvania, Philadelphia.
Karlawish drew on examples from other diseases in articulating some of the ethical policy changes that arise when a disease advances from a clinical-pathological diagnosis to an "actuarial" diagnosis, where the doctor predicts future disease based on subtle clinical signs and biomarker findings. “We speak about the probability of a cardiovascular event and when to intervene to prevent it,” Karlawish said. He envisioned a day where the at-risk state of preclinical AD is as routine as cardiovascular risk calculation and preventive care. Many years of study, and successful disease-modifying treatment trials, lie between today and this future vision. In the meantime, Karlawish urged the HAI audience to respect the general public’s fear of Alzheimer’s, and to approach the transition of predictive biomarker assessments such as amyloid PET into the clinic with caution. Other scientists agreed, cautioning their colleagues to keep in mind that social consequences of a positive amyloid scan, such as problems obtaining insurance policies, should be addressed, not just a person’s ability to handle predictive health information. “We should not get swept up in our enthusiasm for the science,” said Mary Ganguli of the University of Pittsburgh Medical School, Pennsylvania.
How will amyloid imaging spread out from specialty settings? What administrative hurdles will—and should—physicians in various healthcare settings face to get their order for an amyloid scan approved? Will independent commercial clinics spring up that offer amyloid imaging and disclose results to cognitively normal people? Karlawish called on the U.S. network of federally funded Alzheimer's disease research centers to gather data on the impact of amyloid status disclosure. “As neurologists, we need to work out guidelines to protect people from adverse impact,” Karlawish said (see Part 3 of this series).
The influence of a well-meaning crop of research neurologists may be limited, cautioned Norman Foster of the University of Utah, Salt Lake City. “Right now, most people even with dementia, much less cognitively normals, never see a specialist, just their PCP. Most PCPs will not go through a complicated process of evaluating whether and how to disclose. We need clear-cut guidelines.”
As a cautionary tale of the stark difference between the careful use in differential diagnosis in specialized dementia care that Rowe had outlined and the present-day reality of primary care, Foster told of a patient who had come from a PCP with a dementia diagnosis which the patient questioned, and insisted on an amyloid test. Foster refused, assessing the patient clinically and with cognitive tests. His cognition proved to be intact and his "forgetfulness" turned out to have been due to hearing loss. A positive amyloid scan in a primary care or commercial setting could have—either incorrectly or at least prematurely—stuck this person with a diagnosis of Alzheimer’s dementia, Foster said. “Because PCPs are likely to order a scan without determining that the patient has a dementing disease, depression, or a medication-related impairment, we ought to decide what is medically advisable, not wait for society to decide whether they will pay for it,” Foster said.
Others at HAI agreed that, as with any new technology, there was potential for abuse, but emphasized the value to the patient of getting a proper differential diagnosis. “When patients get a diagnosis of MCI, it often leaves them frustrated. The percentages of progression do not satisfy them. Many would prefer to know with greater certainty whether they are dealing with AD, so that they and their families can prepare for the future. We have to factor in the benefit to the patient of a clear diagnosis,” said Gil Rabinovici of the University of California, San Francisco.
To date, an amyloid scan would clarify the diagnosis in some cases (e.g., FTD vs. AD for a person with mild dementia), while in others it would still leave the patient with essentially a risk assessment (e.g., high risk of progressing to AD dementia for an MCI patient), cautioned Bart van Berckel at the Vriije University Medical Center in Amsterdam, The Netherlands.
Finally, the 200+ scientists debated how to interpret weakly positive cases whose amyloid level hovers around the threshold of positivity. In routine clinical practice, physicians will likely use a binary read, which amounts to a thumbs up/down rather than an absolute quantitative measurement of amyloid load (see Part 4 of this series). Stephen Salloway, who generally supports the use of amyloid imaging in the clinic, noted that in some of those borderline cases, the amyloid could be a secondary pathology, not necessarily the reason for the clinical finding at hand. The growing incidence of amyloid positivity with age makes this quite possible. For these cases, a diagnostic hierarchy of how the physician weighs the individual pieces of the patient’s workup remains to be established.
Clinicians in the room easily, perhaps predictably, agreed on one thing: If cost were no limiting factor, they would prefer to see not just an amyloid scan, but also an FDG and MRI scan in many questionable cases.—Gabrielle Strobel.
This is Part 2 of a nine-part series. See also Part 1, Part 3, Part 4, , Part 5, Part 6, Part 7, Part 8, Part 9. Download a PDF of the entire series.
No Available Comments
Thanks to amyloid-binding radiotracers and positron emission tomography (PET), research clinicians can now peer inside the living brain to see senile plaques that were once evident only at autopsy. Now available for research, amyloid PET may be approved for more routine clinical use this year. If that happens, doctors may order amyloid scans for their patients, and some people may demand them. However, it is unclear exactly what a positive scan means for a cognitively normal person, and the evidence for high risk of dementia in MCI patients is still emerging—all while preventive or disease-modifying treatments for AD remain elusive. Should cognitively normal people be told if they test positive for amyloid in the brain? Should they even be scanned? How about people with subtle memory problems? Should only specialists or also primary care physicians be able to order an amyloid scan? Will for-profit clinics sprout up that offer amyloid PET without appropriate safeguards?
Researchers are beginning to put considerable thought into the ethical questions that surround disclosure of a persons’ amyloid status. In light of longitudinal studies that increasingly link brain amyloid to subsequent cognitive decline, they wonder how that emerging knowledge is best handled in the clinic. Questions abound. Who should get a scan and who should be told the results? Is it appropriate to worry people over an illness they may not express for many years and for which there are no good treatment options? Does knowing give some peace to people who already worry because they sense their minds are slipping? If someone is to be told, how can the information be safely delivered? How should potential prevention trial participants be treated differently from patients in routine clinical practice?
At present, none of more than a dozen leading clinician-researchers contacted for this story routinely discloses amyloid status to cognitively normal people. Most researchers agreed that in future, plaque status should only be disclosed when a patient has symptoms, and that any information revealed should be tempered with pre- and post-test counseling. No formal guidelines have yet been issued on the matter, but initiatives are underway to put this consensus in writing in Europe and the U.S. (see below).
However the field decides to deal with disclosure, it needs to do so soon. For now, the original amyloid imaging agent Pittsburgh compound B (PIB) is exclusively used in research studies in which plaque status is almost universally withheld. PIB, labeled with carbon 11, has a short half-life, and is limited in use to centers that have a cyclotron for production and immediate use. But soon, longer-lived agents labeled with fluorine 18 may become available more broadly. One, florbetapir (see ARF related news story), sponsored by Avid Radiopharmaceuticals, now owned by Eli Lilly and Company, is under regulatory review. The company seeks approval of the tracer as a tool to support a clinical diagnosis in people with symptoms of dementia (see ARF related news story), and many researchers expect it to be approved this year.
"Amyloid imaging is probably going to be available soon," said David Knopman of the Mayo Clinic, Rochester, Minnesota. "It would be nice to have a consensus in the expert community about what to say about "positive scans" before that Pandora's box has been opened."
Whom to Tell?
Experts agree that plaque status should be revealed to people who can benefit from the information. Those include the fraction of AD patients who have a questionable diagnosis. Amyloid scans could help support or refute that diagnosis, said Bill Klunk, who co-developed PIB at the University of Pittsburgh in Pennsylvania. As more longitudinal data become available, clinicians may also be able to make better predictions about amyloid positivity in people with MCI, and use the scan to find out whether AD underlies their symptoms. "Most academics would agree that the usefulness of amyloid imaging would be in the differentiation of progressive from non-progressive MCI," said Giovanni Frisoni of the San Giovanni di Dio Fatebenefratelli Hospital in Brescia, Italy.
As a general rule, experts at this point counsel against scans for cognitively normal people. Data from current longitudinal studies, such as the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL), indicate that perhaps a third of cognitively normal people aged 60 and above are PIB-positive (see Rowe et al., 2010). In the ADNI study of older adults, that number is closer to 40 percent (see ARF related news story); numerous other aging cohorts have recently added amyloid imaging and are confirming increased incidence of amyloid positivity with advancing age. However, none of the studies has been ongoing long enough to determine whether these healthy amyloid-positive individuals progress to dementia. Therefore, all experts agreed, it is impossible at this point to make amyloid-based risk assessments for cognitively normal people. "We still need more information about what the implications are of a positive amyloid scan or of abnormal CSF AD markers," said Nick Fox, University College London, U.K. Many cognitively healthy people die of other causes with large Aβ accumulations in their brains. It is not clear whether or how quickly plaques become harmful (see ARF related news story). "Many of us feel that if a PIB-positive person were to live long enough, it would lead to cognitive decline, but we really don't know enough details about that now," Fox added.
Experts are concerned that people who test positive for brain amyloid could overinterpret the predictive value of the results, become depressed, make rash decisions—or even attempt suicide. "It is irresponsible in a clinical setting to infer something about cognitively healthy people's clinical course based on their amyloid status—we just don't have the data to support any conclusion," said Reisa Sperling of Brigham and Women's Hospital in Boston. "We are just at the beginning of our understanding," agreed Michael Weiner of the University of California, San Francisco, who leads the Alzheimer's Disease Neuroimaging Initiative (ADNI). "We have got to work as a community to develop proper guidelines."
The lack of an effective therapy that prevents or slows AD makes scan information even less useful for cognitively normal people. Once a treatment becomes available, the situation will change radically, said Klunk. "Then we'll start screening people, tell the ones who have amyloid in their brains, and suggest that they go on this preventive therapy," he said.
The Paternalistic Doctor?
What if some cognitively normal people are concerned about plaques—either because they have subjective memory complaints or a family history—and demand a scan? Some studies are beginning to show that people with subjective memory complaints are often right in their awareness that something is wrong (e.g., Perrotin et al., 2012). Should they be given a scan and its results? Opinion on this question diverges widely. Most clinician-researchers said they would refuse, but some counter that the patient has a right to know.
On rare occasions, participants who received scans as part of research have insisted on disclosure to the point of threatening to force it through a Freedom of Information Act request, researchers said. "Some individuals are extremely determined; they want to be in control of their future and I don't see why they should be denied knowledge of their brain amyloid status if they want it," said Christopher Rowe, Austin Hospital in Melbourne, Australia. "But it is essential that the significance of a positive versus a negative scan is explained clearly," said Rowe. Careful counseling to explain that a positive scan doesn't mean people will get Alzheimer's and a negative scan does not mean they are immune is crucial, most agreed. In the rare exceptions where clinician-researchers disclose, they couch amyloid positivity as a risk factor, not a deterministic finding. Amyloid status is not disclosed to subjects in the AIBL study, on which Rowe is a lead scientist. Other scientists have made exceptions for cognitively normal people with an autosomal-dominant family history of AD, who insisted on learning their status.
Studies of genetic testing related to ApoE and Huntington's disease suggest that people will not necessarily be flooding clinicians' offices for amyloid scans, said Scott Roberts, University of Michigan in Ann Arbor. Roberts is co-principal investigator on the series of REVEAL studies that examine the psychological and behavioral ramifications of disclosing people's ApoE status. "There's a self-selection process—people who come forward for the information tend to be ready to handle it, and people who are more vulnerable tend to stay away in the first place," said Roberts. REVEAL has found no evidence that people are psychologically harmed by a positive ApoE4 diagnosis (see ARF related news story). "People actually fare better than we often expect, assuming we give proper education and counseling support," he said. At the same time, Roberts conceded that the situation might be different for amyloid scans because brain plaque suggests a more imminent problem.
“People fear Alzheimer’s more than heart disease,” said Jason Karlawish of the University of Pennsylvania Medical School in Philadelphia at the Human Amyloid Imaging Conference held 12-13 January 2012 in Miami Beach, Florida. Karlawish specializes in ethical implications of Alzheimer’s research. “We have to respect that fear, and establish a process to safely and effectively communicate a predictive diagnosis. A stepwise process for disclosure is key.”
In fact, after pre-test counseling, many people decide they do not want to know after all, said John Morris, Washington University School of Medicine, St. Louis, Missouri. Morris does not disclose scan results in his studies, but is exploring whether and how to do that in the future. He asks interested research participants whether they would still want to know after considering, for instance, what the results might mean about family members' risk or the ability to purchase long-term care insurance. "Very often, when we start talking about what the implications are for individuals, they begin to reconsider," Morris said. Requests for results may also be curtailed by health insurance coverage for the scan, which may be limited.
Some researchers have already started disclosing scan results to a few study participants. Hermann-Josef Gertz, University of Leipzig, Germany, revealed plaque status to a handful of cognitively healthy patients who, after counseling, still wanted to know. They all seemed to take the results well and showed no adverse psychological effects, Gertz said. He attributes this, in part, to careful counseling that explained the uncertainty of the test, and that Alzheimer's progresses slowly with long, mild stages. "If we try to convey this view of AD, it may be less frightening," he said.
Another research study at the University of Munich in Germany employs counseling and reveals plaque status to participants with MCI if they wish to know it, said Alexander Kurz, one of the researchers involved in this study. Almost universally, these patients cope well with the results, in part because researchers point them toward interventions that may help somewhat: participation in preventative treatment trials, acetylcholinesterase inhibitors, or compensatory strategies to cope with the memory loss. "It doesn’t cause a lot of anxiety as long as you have something you can do for it," said Kurz.
There are catastrophic exceptions that even a careful process cannot prevent, however. At Kurz’s center, a woman with memory complaints insisted on a CSF biomarker test and disclosure even after going through more than six months of counseling. She committed suicide after receiving a CSF biomarker test that suggested Alzheimer's, Kurz said. The patient had a professional education, and a sister five years her senior was institutionalized with advanced AD. She had cleared an evaluation for depression but did not want to live with the disease. Despite this tragic outcome, Kurz takes the view that, in the end, it is a patient's decision to obtain this information. "Ultimately, every patient has the right to know," he said.
Initiatives to Forge Consensus
Several efforts are afoot to develop a framework for a unified approach to amyloid status disclosure. One headed by Gertz and Pieter Jelle Visser, University of Maastricht, The Netherlands, appears most advanced. These researchers are formulating a consensus statement within the European Alzheimer's Disease Consortium (EADC). The statement will outline for clinicians and researchers who should receive amyloid or biomarker testing in the course of diagnosis, and how the results should be used. Gertz, Visser, Frisoni, and other clinicians have discussed the issue, and Gertz has drafted a paper for presentation at an EADC meeting this coming May. The authors plan subsequent journal publication to help get researchers and clinicians on the same page until formal consensus guidelines become available. "This is an attempt to get a first European outline of future standardization," said Gertz.
More scientific study will be necessary to iron out disclosure issues, said Visser. "What we really need is a trial that discloses—or not—biomarker status to see what happens in terms of outcomes such as psychological well-being," he said. Jennifer Hagerty Lingler of the University of Pittsburgh, Pennsylvania, is heading in this direction. In a pilot trial, she is going through the motions of revealing plaque status to people with MCI, without doing any actual scanning. The patients know that it is a mock reveal. Lingler will conduct patient surveys during this study, asking patients and their study partners such things as, among others: Which counseling methods are most effective? How much information is enough? How well do you understand what you are being told? Do you prefer a study partner to be involved? Based on the results, Lingler plans to conduct a study in which volunteers with MCI undergo actual scans, and in which she uses her previously established methods to reveal their amyloid status. She plans extensive follow-up to monitor people's psychological state and evaluate potential benefits and drawbacks of disclosure.
"It's very important to prepare the field for managing disclosure sessions and delivering information in a comprehensible way," Lingler said.
This issue saw considerable discussion at the Human Amyloid Imaging Conference held 12-13 January 2012 in Miami, Florida. “The guiding principle that we have no obligation to disclose amyloid status so long as criteria for preclinical AD don’t directly affect clinical care will become less and less feasible in the near future,” Karlawish told the audience at HAI.
One such challenge awaits researchers for upcoming studies in which they intend to recruit cognitively healthy, amyloid-positive people into preventive anti-amyloid trials. Sperling and colleagues are preparing for one such study, the A4 trial (see ARF related news story), in which people will—by way of their participation—learn their amyloid status. “If you do a spinal tap or a PET scan to find out amyloid status and then are asked to join the study, you know your status,” Karlawish said. Sperling has partnered with Karlawish to come up with a standardized approach for disclosing amyloid status for A4 in an ethical and safe way. Designs using blinded placebo default groups avoid that, but they have practical disadvantages.
"We need to set up a process to assure that the information that participants get is understandable and is given in a way that minimizes harm," Karlawish told Alzforum. He and his colleagues will draw on best practices from genetic counseling for carriers of genes such as the ApoE4 allele, and do their own research as well. Assuming funding will come through, they hope to have a plan ready by early 2013.
With these initiatives to build consensus around how to bring amyloid PET—and diagnostic biomarkers in general—to patients, the community is trying to come to grips with the reality of a growing and expensive diagnostic armamentarium entering clinical care faster than disease-modifying therapies. “I predict that, as advances are made in AD therapeutics, disclosure practices will become less intensive,” Karlawish said at HAI. “Consider HIV/AIDS. Progress in treatments has led to more relaxed HIV testing guidelines, even home testing. You can now order the test and get your results over the phone. Eventually, that can happen in AD, too.”
But for now, these are early days. "There is no standard for disclosure whatsoever because we are all taking the first steps," said Gertz. "We are looking for a standard, trying to discuss and to find a common base, but we are very much at the beginning."—Gwyneth Dickey Zakaib.
This is Part 3 of a nine-part series. See also Part 1, Part 2, Part 4, , Part 5, Part 6, Part 7, Part 8, Part 9. Download a PDF of the entire series.
No Available Comments
For amyloid imaging to become widely useful in clinics beyond a small number of research settings, a nuclear medicine physician should be able to look at a person’s scan and know if the scan is positive or negative. No number crunching, no data plots—just a quick, so-called binary read. That instantaneous interpretation comes with an intangible human element. Can it be done correctly and reliably? This question came up last January at an FDA advisory committee meeting. There, concern over variability among readers prompted the FDA to direct the radiotracer’s sponsor first to develop a reader training program and prove that it works before requesting that florbetapir be approved for clinical use (see ARF related news story). At the 6th Annual Human Amyloid Imaging Conference held 12-13 January 2012 in Miami, Florida, Mark Mintun of Avid Radiopharmaceuticals in Philadelphia, Pennsylvania, presented data to address this charge (Mintun et al., 2012).
Minton and colleagues developed a binary read method for florbetapir scan images displayed in black and white. The method is based on visually judging the extent of contrast at the brain’s white matter-gray matter boundary. For a scan to be called positive, it has to have reduced contrast along an inverted gray scale in at least two brain areas. Mintun presented a visual read and testing program where the goal was to call correctly whether the scan is positive or negative. The scientists used several series of scans; some had predetermined right or wrong answers based on postmortem pathology read with CERAD criteria for the frequency of neuritic plaques, while other series were from clinical practice and judged against the clinical diagnosis.
The training starts with a lecture; then, the physicians practice on five demonstration cases and seven practice cases in an interactive session, and then the physicians assess what they have learned on 20 more cases. This takes three hours, either in person with a trainer present or remotely with a DVD. Mintun and colleagues tested this training regimen in three studies, one using 35 autopsy cases and nine readers, one using 59 autopsy cases and five other readers, and one using 59 autopsy cases on five more readers. The median sensitivity and specificity was in the low 90s, Mintun reported. How well one reader agrees with another is typically expressed by a measure called Fleiss’ kappa; 1.00 signifies perfect agreement, and in this series of tests it came in at 0.85 to 0.75.
In-person training yielded slightly better results than did remote training, where the physicians clicked through the material but did not have an expert in the room to ask questions. The nuclear medicine physicians came from academic and private practice backgrounds and did not have to have experience with brain PET, Mintun told the audience. When readers called a scan incorrectly, it tended to happen on the same brains. For example, one positive case had died two years after the scan, and most readers read it as negative; other readers had trouble with atrophy or when the signal seemed to them to be right on the border, Mintun said during audience discussion.
Minton noted that when non-autopsy cases of clinically diagnosed Alzheimer’s, mild cognitive impairment, and controls were mixed into an autopsy series, agreement among the readers rose. He addressed a concern that had arisen a number of times in previous sessions at HAI, that is, whether MCI might be harder to read and generate more borderline results. “In our reader training, that was not true,” Mintun said. Reader agreement for 92 clinical MCI cases was 98 percent, Mintun reported, adding that the readers also expressed more confidence reading clinical MCI cases than interpreting the autopsy cases. Autopsy cases can seem ambiguous because they show brains of people who were very ill at the time of their scans, Mintun said in discussion. Others countered that, while clinical research cases of MCI may indeed be less ambiguous than autopsy cases, day-to-day patients in routine clinical settings may be trickier to read because they may have strokes, white matter disease, and other comorbidities. Overall, though, the audience at HAI saw the results of this training program as reassuring.
Importantly, nuclear medicine researchers tend to pursue a different goal than the FDA, which acts on behalf of the general public being treated by non-specialist clinicians. Researchers prefer quantitative, nuanced measurements, whereas the FDA wants to be convinced that a robust, thumbs up-down binary read will serve the public, said Keith Johnson, who co-organizes the HAI Conference. At the conference, several presentations explored ways of setting appropriate thresholds above which to call a scan positive. For example, Ann Cohen of the University of Pittsburgh Medical School compared threshold-setting methods and tested them with a handful of independent, blinded readers (Cohen et al., 2012). Gil Rabinovici of the University of California, Berkeley, compared more liberal and more stringent published thresholds in a pathology series of early-stage patients to check whether any might be set too low. He reported that even with the liberal threshold, once people’s scans read positive, they already had abundant β amyloid in their brains, alleviating concern about potential false positives (Rabinovici et al., 2012).
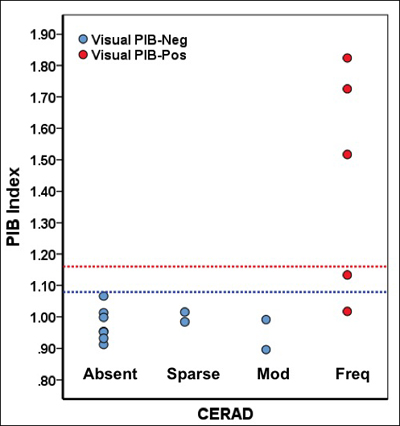
Relationships among postmortem CERAD diagnosis, quantitative PIB threshold (blue line = liberal, red line = conservative), and visual reads. All scans read as positive showed frequent CERAD plaques. Image credit: Gil Ravinovici, William Jagust
A slew of posters showcased academic-industry collaborations to formally standardize amyloid PET for robust performance in multicenter studies. Overall, scientists agreed with Robert Koeppe’s advice that the field will need both visual reads and quantitative analysis. The former allows an up-down determination of whether a scan is positive, while the latter can pick up early-stage deposition in individual regions and subtle changes over time or in response to drug.—Gabrielle Strobel.
This is Part 4 of a nine-part series. See also Part 1, Part 2, Part 3, Part 5, Part 6, Part 7, Part 8, Part 9. Download a PDF of the entire series.
No Available Comments
At the Human Amyloid Imaging Conference, held 12-13 January 2012, three speakers presented data on brain amyloid deposition in presymptomatic carriers of deterministic Alzheimer's disease mutations starting as early as their young twenties. Two datasets came from large cohorts and were similar, while one came from a small group that appears to represent an exception to the rule.
Adam Fleisher of the Banner Alzheimer’s Institute in Phoenix, Arizona, presented florbetapir pilot data of the Alzheimer’s Prevention Initiative Biomarker Project. The API-BIO aims to characterize, compare, and order the emergence of the currently available major biomarkers of AD pathogenesis in Colombian families who carry the E280A Paisa mutation in presenilin-1. This population represents the world’s largest known kindred of any AD mutation, and API is the largest study to date of autosomal-dominant AD. In preparation for multiple future clinical trials, the project is tracing back at what age each biomarker candidate—amyloid imaging, FDG-PET, MRI, fluid markers, cognition—first diverges between a carrier and their non-carrying siblings. At HAI, Fleisher presented initial data from September to December of 2011, when API colleagues in Colombia and Phoenix worked furiously to enable five successive groups of a total of 50 study participants and their relatives to travel from Medellin to Bogota to obtain visas, and then through Miami on to Phoenix for brain imaging. “For many of them, it was the first visit to Bogota, not to mention their first air travel and trip to the U.S.,” said Fleisher.
Each group stayed in Phoenix for several days before returning to Colombia. There they received a florbetapir and an FDG-PET scan. Banner staff, Avid Radiopharmaceuticals, and Cardinal Health made radioligand and scanners available through the weekend, while API staff supported the study volunteers during their stay, Fleisher said. Natalia Agudelo, a young Colombian woman who had been featured in a New York Times story, and whose father passed away of AD last year, became the first Colombian to receive a scan with florbetapir, the tracer used in this study.
The 50 participants were 18 to 60 years of age with a mean age of 32, matched for sex and education, and grouped to contain equal numbers of carriers and non-carriers. Nineteen carriers were cognitively still normal; 11 were symptomatic. Overall, the scientists saw a pattern of florbetapir uptake similar to that seen with florbetapir PET in late-onset AD cases, Fleisher told the HAI audience. When inspecting the scans visually, carriers started being positive for fibrillar amyloid in their precuneus, parietal cortex, and striatum around age 30, some 15 years before the age of mean symptomatic onset in the Paisa mutation families. Quantitative measurement of uptake across the cortex started detecting amyloid a bit earlier, around age 28. From there, uptake grew in a sigmoidal curve until age 37 to 40, and then reached a plateau as carriers entered the symptomatic stage of their disease (Fleisher et al., 2012).
Tammie Benzinger of Washington University, St. Louis, Missouri, presented data of 100 of the participants enrolled to date in the Dominantly Inherited Alzheimer Network (see ARF related news story). DIAN is the largest cohort worldwide of families with familial AD in the U.S., Australia, and the U.K., totaling more than 40 different mutations in the APP and presenilin genes. As in the case of API, the cohort is young, with a mean age of 35. Also as in API, the overall amyloid PET finding is that presymptomatic carriers have a gradual buildup of amyloid in the same brain areas as known from late-onset AD. “The carriers are different from the non-carriers in every gray matter area we tested,” Benzinger told the HAI audience (Benzinger et al., 2012)
Not all is the same between DIAN and API, nor between eFAD and LOAD, however. For one, in concordance with current thinking on LOAD, the DIAN study detects changes in FDG-PET and volumetric MRI later, not until carriers are mildly symptomatic with a 0.5 on the Clinical Dementia Rating Scale, whereas API has reported seeing subtle decrements on functional and structural imaging earlier than that (see ARF related news story). For another, there is quite a bit of variability between the studies in where amyloid first crops up—it can be the basal ganglia in some cases, the frontal lobe in others. API does not see the early striatal uptake reported in the first cases of PIB-PET in familial AD (e.g., see ARF related news story), confirming researchers' hunch that this much-discussed finding might be specific to certain mutations. DIAN sees early uptake in some areas that aren’t on the typical list of affected regions for LOAD, such as the occipital cortex and the orbital frontal lobe, Benzinger reported. At 20 to 25 years prior to expected onset, DIAN is finding PIB positivity a tad earlier than API. This could either have to do with greater sensitivity of PIB for small gray matter signals or reflect the heterogeneous mutations represented in DIAN.
That said, Benzinger and Fleisher agreed that their studies’ overall amyloid PET findings at this early stage match up well. DIAN contains a more heterogeneous set of patients than API, some of them having significant cerebral amyloid angiopathy along with their AD. The data are but an initial cross-sectional look thus far, and cannot be truly compared to each other yet. “We need longitudinal data, more participants, and then really drill down,” said Fleisher. Only then will scientists know which differences are real, which are large, and which are minor. Meanwhile, “whether amyloid imaging will eventually show eFAD to be substantially similar or different from LOAD remains an open and important question,” said Keith Johnson of Massachusetts General Hospital.
While most AD mutations look at least similar on amyloid PET scans, one appears to break ranks. At HAI, Agneta Nordberg of Karolinska Institutet in Stockholm, Sweden, presented data showing that the very rare so-called Arctic mutation of APP is essentially a no-show on PIB-PET scans. Known only in one Swedish family and a descendent U.S. family, this mutation causes an early onset form of AD that is clinically like sporadic AD but pathologically quite a different story. Characteristic plaques seen in postmortem LOAD with standard stains for fibrillar deposits, such as Congo red, come up negative in tissue with the Arctic mutation. At HAI, Nordberg reported that five carriers of this mutation, four of them presymptomatic, were negative for PIB retention. This means that PIB-PET matches up with postmortem pathology; hence, technically speaking, amyloid PET works, Nordberg said. More broadly, however, it means that forms of amyloid that are invisible to PIB (and presumably its 18F cousins) can cause clinically typical AD, too.
This form of AD behaved as expected in other biomarkers tested, Nordberg reported. In FDG-PET, the carriers showed the AD-typical deficit in frontoparietal, temporal, and posterior cingulate cortex; MRI revealed increasing atrophy going from cognitively normal carriers to the AD patient; and CSF markers of Aβ42 and tau/p-tau were severely abnormal, as in sporadic AD. “All biomarkers fit the picture; just fibrillar Aβ PET is aberrant with this mutation,” Nordberg said (Nordberg et al., 2012).
These data suggest, to Nordberg’s mind, that other forms of Aβ, for example, oligomers and protofibrils, can cause the pathological processes leading to AD. Other researchers agreed that this study highlights the need to develop tools to capture those other Aβ species. How many cases like these might be out there? Besides the Arctic mutation, a Japanese deletion mutation that behaves similarly has been described, as well as some isolated cases of PIB-negative LOAD. The phenomenon seems rare, but researchers don’t really know the extent of it.—Gabrielle Strobel.
This is Part 5 of a nine-part series. See also Part 1, Part 2, Part 3, Part 4, Part 6, Part 7, Part 8, Part 9. Download a PDF of the entire series.
No Available Comments
At this stage of Alzheimer's disease research, scientists know that APP or presenilin mutations and the risk gene ApoE4 have two things in common. Both bring down the carrier’s age of disease onset, ApoE4 less drastically than the deterministic mutations. Also, both types of genetic risk push brain amyloid deposition back to younger ages. At the Human Amyloid Imaging Conference, held 12-13 January 2012 in Miami, Florida, several labs presented new data on the connection between ApoE and amyloid deposition in preclinical AD and aging. They are trying to pinpoint when it first deposits and when it might start affecting cognition.
First off, however, a counterpoint to ApoE4. The ApoE2 allele is known to protect against Alzheimer’s, but because it is rare, few studies have been able to look at its mechanism. At HAI, Eisuke Haneda of the Tokyo Metropolitan Institute of Gerontology, Japan, and colleagues reported data from a joint analysis of U.S.-ADNI, the Japanese ADNI, and the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL). Together, these three studies count at least 24 people with ApoE2 among them. On a poster, Haneda showed that homozygous and heterozygous ApoE2 carriers were much more likely to be amyloid negative in their respective study’s baseline scan than ApoE4 carriers, and even those who did have some amyloid had less of it in the cortex and precuneus (Haneda et al., 2012).
Presenting the first of many studies on ApoE4, Christopher van Dyck of Yale University of Medicine in New Haven, Connecticut, ran PIB scans on cognitively normal volunteers in their fifties and early sixties who had a parent with AD. His study was small—15 people each for the ApoE4/4, 4/3, and 3/3 genotypes. (Like ApoE2, ApoE4 homozygosity is rare, making study participants hard to find.) In this first cross-sectional comparison, PIB uptake was higher in people with the 4/3 genotype than in the 3/3s, and higher still in the 4/4s. The youngest person with significant PIB uptake was a 51-year-old homozygous E4 carrier. In this age group, van Dyck’s group was unable to detect any effect of their amyloid positivity on neuropsychologic performance. That said, most tests used in this study have their greatest dynamic range later, when people are already cognitively impaired, and hence might not have picked up subtle decrements in this age group. Longitudinal studies of this cohort might reveal an association with cognition later on, scientists noted during subsequent discussion (van Dyck et al., 2012).
Two studies presented longitudinal data on cognitively normal cohorts genotyped for ApoE, though these study volunteers are a bit older. Jessica Langbaum of the Banner Alzheimer’s Institute in Phoenix, Arizona, reported two-year PIB-PET data on cognitively normal people in their sixties. This group includes eight ApoE4/4 carriers. Two years after their initial scan (Reiman et al., 2009), they were still cognitively normal, Langbaum said. PIB uptake was again markedly higher in E4 homozygotes and heterozygotes than E4 non-carriers, and it had increased from the baseline scan. In particular, β amyloid accumulated in the prefrontal, lateral, parietal, and precuneus regions of the brain. Langbaum noted that once a person has brain amyloid deposition, it is there to stay. Indeed, multiple scientists doing their own longitudinal studies agreed that, unlike a diagnosis of MCI, where a fraction of patients revert to normal cognition some years later, preclinical amyloid deposition stays and grows in all people repeatedly scanned thus far (Fleisher et al., 2012).
A third study in cognitively normal people offered a breakdown of amyloid deposition by ApoE status. Andrei Vlassenko of Washington University School of Medicine, St. Louis, Missouri, reported changes in between two PIB scans that were, on average, 2.6 years apart. This study included 146 people aged 45 to 86 who were cognitively normal with a CDR of 0 at both time points. Of them, 21 were positive at the first scan and stayed positive at the second scan, whereas 115 were negative at the first and still negative at the second scan. Ten people crossed the threshold from negative to positive between scans. Recently, the scientists started third scans in this cohort. Of 12 people analyzed so far, 11 remain amyloid negative; one person who had previously crossed the threshold demonstrated continuing β amyloid accumulation in a linear fashion, Vlassenko said. In these people, scientists in essence can catch the first glimpses of fibrillar amyloid deposition in a person’s life. The average age of this group was 65; the youngest converter was a 56-year-old man with two copies of ApoE4. Seven of the 10 converters were ApoE4-positive, though it should be noted that this sample is enriched for ApoE4 carriers.

Repeat measurements show brain Aβ levels cross the threshold (red) and increase with age in people with (black) or without (blue) ApoE4. View larger image. Image credit: Vlassenko et al., 2011
With these numbers, the scientists can begin to calculate incidence of conversion. It came out to 3.1 percent for the whole group, and 7 percent for ApoE4 carriers, though these numbers may well change as more people in narrower age ranges have longitudinal scans. Part of these data recently appeared in Annals of Neurology (Vlassenko et al., 2011). "For the first time in the literature, we describe the conversion from no preclinical AD to preclinical AD," said Vlassenko. At HAI, scientists at Avid Pharmaceuticals, the Philadelphia-based company that develops florbetapir, reported a similar 3.5 percent annual conversion rate of people who were amyloid negative at baseline but positive in a subsequent scan.
Where did the amyloid first crop up in these converters? “It’s really quite broad,” Vlassenko said, with highest retention seen in posterior and anterior cingulate, precuneus, lateral and medial frontal cortex. Most people showed an increase in their PIB retention between the first and subsequent scans, which fits with the increase in fibrillar amyloid seen in early MCI (Vlassenko et al., 2012).

PIB distribution on lateral and medial surfaces of the cortex on the left and right hemispheres of the human brain. A, Healthy adults younger than 50 y; B-D, Cognitively normal adults older than 50 with low (B), moderate (C), and high (D) Aβ deposition; E, Individuals with Alzheimer's dementia. View larger image. Image credit: Vlassenko et al., 2012
The question of when in life amyloid plaques begin to settle in the brain was the subject of a talk by Ozioma Okonkwo at the University of Wisconsin School of Medicine in Madison. Okonkwo analyzed data from 200 cognitively normal people, mostly in their fifties and sixties, who participate in a multimodal imaging substudy of the Wisconsin Registry for Alzheimer’s Prevention, a larger longitudinal study of about 1,500 middle-aged and older adults. PIB scans available so far on 156 of these 200 people show that at baseline, 28 percent of them were amyloid positive. The posterior cingulate, precuneus, and other cortical midline structures were the most susceptible to amyloid aggregation, Okonkwo said. In this sample, although some people younger than 55 years old were amyloid positive, clear-cut elevation in amyloid accumulation appeared to begin at age 55. Here, too, ApoE4 was the strongest predictor of amyloid positivity, with 54 percent of the amyloid-positive participants carrying at least one allele, versus 27 percent of the amyloid-negative participants (Okonkwo et al., 2012). This study also is enriched for ApoE4 relative to the population at large.
Furthermore, Okonkwo noted that fibrillar amyloid aggregation was associated with cortical thinning and hippocampal atrophy. Surprisingly, higher amyloid deposition was also associated with elevated cerebral metabolism and better performance on certain memory tests, perhaps as a reflection of compensatory processes at this cognitively normal stage. MCI studies have previously noted hypermetabolism by FDG-PET in early MCI, versus hypometabolism by late MCI.
Okonkwo noted that the WRAP study focuses on the effect of family history, and, consequently, he looked into what having a parent with AD did to a person’s chances of having brain amyloid in late middle age. Surprisingly, in this initial analysis of a relatively young cohort (mean age was 59), family history did not significantly affect amyloid deposition. On this question of family history, Jacqueline Maye of Massachusetts General Hospital added data into a mysterious new line of research. Some recent studies have observed that a mother’s Alzheimer’s is a more ominous portent than a father’s for an adult child’s prospect of getting the disease, though other studies have not found this. Moreover, some studies have detected a more AD-like imaging pattern in cognitively normal middle-aged people whose mothers had AD. How amyloid PET might enter into this picture is unclear.
Maye looked at this question in 102 cognitively normal or mildly impaired participants in the Harvard Aging Brain Study in whom she was able to determine at what age the parent had become symptomatic with dementia that was likely due to AD. For 32 of them, their mothers had had AD, in 14, the fathers. What did their PIB scans show? This study required controls on many sources of variation, including the child’s age, gender, CDR status, ApoE status, and education. When all this was done, the data still showed that PIB retention was higher in adult children of demented mothers than either children of demented fathers or children without a family history of dementia. Particularly worrisome for those adult children of affected mothers might be that the earlier in life the mother got dementia, the more pronounced was the effect of higher PIB retention in the adult child. This effect held true for sons and daughters, and it is separate from ApoE, Maye said (Maye et al., 2012).
Still more normal aging studies elsewhere have added amyloid imaging to how they assess their cohorts. At HAI, Gerard Bischof at the University of Texas at Dallas teased apart the effects of brain amyloid and white matter hyperintensities in the Dallas Lifespan Brain Study. This is a sample of aging people selected for their robust overall health, i.e., they have no cardiovascular disease, diabetes, or other common diseases of aging. In the 89 people aged 50 and up whom Bischof analyzed, amyloid burden not only increased with age, but each type of brain lesion also appeared to do its independent share of damage to the brain. White matter changes affected reasoning and, perhaps surprisingly, episodic memory, whereas β amyloid as shown by florbetapir hampered working memory, executive function, and processing speed (Bischof et al., 2012).
Studies across continents have been finding a link among ApoE status, age, and brain amyloid with such consistency that some researchers are even entertaining the idea of viewing ApoE and age as a proxy for brain amyloid. This could potentially save costs for secondary prevention trials, noted Michelle Mielke of the Mayo Clinic in Rochester, Minnesota. Mielke reported that in the Mayo Clinic Study of Aging, a population-based normal aging study, 483 people age 70 and older have had PIB-PET scans, and about a third them were positive. Mielke calculated how well other AD risk factors captured in this study might predict PIB positivity as a way of helping secondary prevention trials enrich for brain amyloid without having to go to the expense of screening large numbers of people with PET imaging. Predictably, perhaps, age and ApoE genotype came out on top, suggesting that these two factors can flag the likely presence of brain amyloid (Mielke et al., 2012). Cognitive performance or family history added little in the way of predictive value, Mielke reported.
What about people in their eighties and nineties? In general, scientists poorly understand the role of amyloid in the very old, and this knowledge gap was widely discussed at HAI. The oldest people in Vlassenko’s study, for example, were in their eighties; they appeared to form an exception to the perceived rule of amyloid deposition growing or staying stable over time in that their amyloid retention appeared to dip a bit from one scan to the next. But even among the oldest old, amyloid deposition appears to be bad news. Claudia Kawas of the University of California, Irvine, in a lecture on dementia in the oldest old, showed florbetapir imaging on 13 participants in the longitudinal, population-based 90+ Study. One person who underwent an amyloid PET scan was 99.9 years old, Kawas said. Against her expectations, Kawas found that people who had low amyloid uptake on their scan remained cognitively stable over the 1.5 years of follow-up data available so far, whereas the four participants who had high uptake declined steeply over the course of the next year. “I was shocked by this,” Kawas said. This appears to contradict the idea that amyloid is less related to dementia in the oldest old than in younger people (see, e.g., ARF related news story).
"It's very important that we learn as much as possible about what is going on in the brain before cognitive symptoms arise," said Nordberg. "That will give us a lot of information to really understand the time course of pathological changes." There was wide agreement that larger numbers of people will need to be monitored for at least 10 years with periodic amyloid PET and other risk factor and biomarker assessments before scientists can predict who is going to develop AD and when. In the meantime, there is already broad agreement that cognitively normal or minimally impaired people with amyloid deposition are becoming an important population for early-stage intervention trials.—Gabrielle Strobel.
This is Part 6 of a nine-part series. See also Part 1, Part 2, Part 3, Part 4, Part 5, Part 7, Part 8, Part 9. Download a PDF of the entire series.
No Available Comments
At the 6th Annual Human Amyloid Imaging Conference, held 12-13 January 2012 in Miami, Florida, researchers updated each other on what their respective longitudinal studies were showing. By and large, their studies appear to support the notion that amyloid deposition heralds future cognitive decline.
Chris Rowe of the University of Melbourne, Australia, presented two-year data on 45 people who had mild cognitive impairment at baseline and were followed with clinical and neuropsychological testing every six months and an amyloid PET scan every year (Ong et al., 2012). This study used florbetaben, an 18F radiotracer that is roughly equivalent to florbetapir and flutemetamol, according to scientists. At baseline, half of the participants had high florbetaben retention; at that time, their composite memory scores, even their MMSE, tended to be lower. Two years later, their florbetaben retention had grown by 3.1 percent, Rowe noted. Importantly, 79 percent of them had progressed to Alzheimer’s dementia, whereas 24 percent of the people with low florbetaben retention at baseline had progressed to other diseases such as frontotemporal dementia or progressive supranuclear palsy. “We see a very strong correlation between baseline florbetaben levels and subsequent memory decline,” Rowe said.
In this study, Rowe directly compared MRI and florbetaben PET for their ability to predict progression from mild cognitive impairment (MCI) to Alzheimer’s dementia (AD). Besides their amyloid scans, the study participants also received an MRI scan, and the Australian researchers visually scored those with two published academic methods and an FDA-approved commercial program called NeuroQuant. The upshot? The amyloid scan outperformed all three ways of reading the MRI in specificity and in overall accuracy, Rowe reported. Within the methods of MRI interpretation, the commercial program was most accurate, Rowe added.
This study addressed one more wrinkle about MCI. When splitting the group into early (eMCI) and late patients, a gap opened up. Of the late MCI cases, 79 percent were amyloid positive at baseline, and of the eMCI cases, only 40 percent. When applying the same comparison of prediction by florbetaben versus by MRI to these groups separately, the researchers found that amyloid PET outdid the MRI measures in eMCI by far more than in late MCI. Amyloid PET is more valuable in the early MCI group, largely because late MCI itself already appears highly specific for AD, Rowe said. This mirrors similar data by Cliff Jack’s group at the Mayo Clinic in Rochester, Minnesota, who previously reported that the predictive ability of amyloid scans flattens out somewhat as it nears its plateau (Jack et al., 2010). Trialists at HAI said that amyloid imaging still should be included in selecting patients at all stages of MCI for multicenter trials, because clinics vary widely in how they apply MCI criteria, and some have low conversion rates.
Similar results came out of a different two-year study presented by Michael Pontecorvo of Avid Radiopharmaceuticals. All coauthors of this study are employees of Avid. Thirty-six people with MCI and 49 cognitively normal people underwent a florbetapir scan at baseline and once again some 23 months later. First author Abhinay Joshi and colleagues found that, at baseline, 44 percent of the former and 20 percent of the latter were amyloid positive (Joshi et al., 2012). The cognitively impaired participants in this study are eMCI, with a CDR of 0.5 and an MMSE above 24, and hence match up well with the 40 percent florbetaben positivity Rowe found in the Australian eMCI volunteers, Pontecorvo noted in his talk. The amyloid-positive people had about 3 percent more amyloid the second time around, a small increase that cannot be seen visually but requires quantification. No amyloid-positive person reverted. Most amyloid-negative MCI patients stayed negative two years later. “They are not on the path to AD,” Pontecorvo told the audience.
On a poster with three- to five-year data from AIBL, Rowe’s colleague Victor Villemagne showed that among 118 cognitively normal participants, those with faster amyloid deposition as measured by PIB declined significantly faster on memory tests than did fellow participants with slower or no amyloid deposition (Villemagne et al., 2012). The same was true for people with MCI and, interestingly, AD patients. Similarly, Miranka Wirth of the University of California, Berkeley, presented a poster suggesting that, among 38 cognitively normal older people undergoing three annual neuropsychological exams, those who were positive for PIB declined on memory. Susan Landau at UC Berkeley looked at amyloid deposition and subsequent cognitive function in 325 ADNI participants across the spectrum of normal, early, and late MCI and AD who had had florbetapir scans. In this sample, too, numbers were similar, that is, amyloid positivity in 30 percent of normal, 43 percent eMCI, 66 percent late MCI. Florbetapir-positive volunteers declined more steeply on episodic memory than florbetapir-negative fellow volunteers. The decline was pronounced in people with MCI and subtle but detectable in cognitively normal people with amyloid deposition, Landau reported (Landau and Jagust, 2012).
Alex Becker of Massachusetts General Hospital is working to parse out how amyloid deposition affects synaptic activity over time by analyzing both amyloid and FDG-PET scans taken about two years apart in cognitively normal and mildly impaired study participants. Becker analyzed data from ADNI1 and the Harvard Aging Brain sample, for a total of 105 cognitively normal and 166 mildly impaired people. Becker looked not at group differences but at declines within a given person from baseline to two years later. He found that, in cognitively normal people, whether a person had amyloid deposits did not affect their age-related decline in glucose metabolism over the next two years. In contrast, people with MCI not only started out with lower metabolism if they had amyloid than if they did not, but their glucose metabolism also declined more steeply over the next two years. Becker suspects that the apparent lack of a relationship in cognitive normals at this point in the analysis may be an artificial null result that results from increases in some brain areas and decreases in others (Becker et al., 2012).
Overall, most available longitudinal studies to date of normal and mildly impaired people appear to tell the same story. “One big thing I learned is that, with the longitudinal studies, we are getting much better convergence between studies than when we looked at cross-sectional data before. That is a great development,” said Sue Resnick of the National Institute on Aging.—Gabrielle Strobel.
This is Part 7 of a nine-part series. See also Part 1, Part 2, Part 3, Part 4, Part 5, Part 6, Part 8, Part 9. Download a PDF of the entire series.
No Available Comments
At the Human Amyloid Imaging Conference, held on 12-13 January 2012 in Miami, Florida, the overriding story was that new data on amyloid imaging are largely confirming the key issues of its neuropathological correlation and its predictive power for cognitive decline. Remaining scientific debate on those fundamentals is moving on to finer points. And yet, underneath that fundamental accord, there were plenty of question marks. For example, on the clinical application of amyloid imaging and other biomarkers, scientists are confronting mismatches that leave them puzzled for the time being. In particular, amyloid imaging appears to contradict the clinical diagnosis of a significant fraction of patients, leaving the clinician-researcher to wonder whether the doc or the scan got it right. This question will find its answer in longitudinal observation, but in the meantime, amyloid biomarkers as currently incorporated into new diagnostic criteria leave the practicing physician with considerable uncertainty, researchers agreed.
Take, for example, the Alzheimer’s Disease Neuroimaging Initiative, (ADNI). Analyzing the data of this flagship study, several groups of scientists are discovering that some of its participants clinically diagnosed according to standardized criteria with probable AD might have something else. Either they are misdiagnosed or current biomarkers appear not to be worth their salt. At HAI, Susan Landau of the University of California, Berkeley, told the audience that, in a study addressing the separate topic of how amyloid and FDG-PET compare, she noticed to her surprise that 22 percent of clinically diagnosed AD patients from her ADNI sample were negative on their florbetapir scan. Similarly, Norman Foster of the University of Utah, Salt Lake City, reported on a poster comparing amyloid and FDG-PET in ADNI that he, too, found that 21 percent of his sample of 70 "probably AD" subjects were negative on their PIB or florbetapir scan (Foster et al., 2012). This, in Foster’s mind, raises concerns about the accuracy of the clinical diagnosis in ADNI. This is true in primary care, as well, Chris Rowe added in a subsequent discussion. Up to a third of people referred to Rowe’s center at Austin Hospital near Melbourne, Australia, with a diagnosis of AD turn out to be negative for amyloid PET, Rowe said.
Scientists want to understand where this discrepancy comes from. Does the problem lie with technical aspects of the PET scan, or with the clinical diagnosis? One way to answer this question is to follow "discordant" patients over time. Pascual Sanchez-Juan at the University of California, San Francisco, showed on a poster what happened to 15 patients at the UCSF Memory and Aging Center over the course of four years after the discordance appeared. Six of 69 people clinically diagnosed AD patients had a negative PIB scan, and nine of 65 people clinically diagnosed with a frontotemporal lateral dementia (which are non-amyloid diseases) had a positive scan. After that, these patients returned repeatedly for further assessments.
Of the PIB-negative AD cases, two stayed stable and their diagnosis changed to MCI due to psychiatric and vascular causes; in other words, the scan appears to have been right. Ditto for three more who evolved to an FTLD syndrome and had their diagnosis changed. One patient, however, continued losing memory and kept the diagnosis of AD; in this case, the scan might have been wrong. Of the nine PIB-positive FTLD cases, four progressed on a typical FTLD course, keeping their original diagnosis. Because they had brain amyloid, doctors prescribed cholinesterase inhibitors for them. Here, the amyloid deposition could have been incidental to their FTD. The five other PIB-positive FTLD patients evolved clinically toward AD, and their diagnosis was changed to AD; three added cholinesterase inhibitors (Sanchez-Juan et al., 2012).
Beyond this data presentation, the issue of how to weigh clinical diagnosis versus biomarker result when the two don’t match sparked intense discussion. Some scientists tended to place more trust in the biomarker, whereas others leaned toward the clinical finding. Attendees agreed that the issue needs to be clarified before diagnostic biomarkers can be widely implemented, which will require more standardization and defined cut points. Many expected that amyloid deposition may eventually sit at the top of a diagnostic hierarchy because it is seen to be the only biomarker that is specific to AD, but there was general consensus that it is too early to draw this conclusion. More data are needed.
As it is, biomarkers have been incorporated for the first time in the recently revised diagnostic criteria. That allows scientists to test how the new criteria perform in existing datasets. At HAI, Val Lowe of the Mayo Clinic in Rochester, Minnesota, presented one such exercise. He noted that the criteria contain a category called “Dementia Unlikely Due to AD” and applied it to ADNI data. In clinical practice, the diagnosing physician would invoke this category when a demented patient is negative for biomarkers of amyloid and downstream neuronal injury. How about it? In Lowe’s analysis, 9 percent of clinical AD cases in the ADNI sample he analyzed were negative for amyloid. Of those, some were negative for neuronal injury markers as well, and hence would be diagnosed as having dementia unlikely due to AD. However, some were negative for one set of biomarkers and positive for the other; those people are not captured at all by the new criteria as currently published, Lowe said (Lowe et al., 2012).
“It is pretty upsetting that these highly clinically selected, supposedly typical AD cases by the new criteria would either be indeterminate or have dementia not due to AD,” said Foster, “I would like to institute these criteria in my practice, but find it is quite often indeterminate.” Rowe agreed, noting, “It happens that I think patients have typical AD, and then they are negative on amyloid PET. I have to tell them I really do not know what they have.” Pathologists across the field have, of course, been noting for years that a fraction of people who died with a clinical diagnosis of probable AD turn out upon autopsy to have had either multiple mixed pathologies or no AD pathology at all. And at last year’s Alzheimer’s Association International Conference in Paris, Nigel Cairns of Washington University, St. Louis, Missouri, noted that, of the ADNI AD cases that have so far come to autopsy, 40 percent meet McKeith diagnostic criteria for dementia with Lewy bodies. In the past, this reality check simply never made its way back to the clinician during the patient’s lifetime, but now there is a technique to image at least one pathology.
Where does this leave the field? More biomarker data may well lead to further revision of the diagnostic criteria. In the process, there may be an unexpected upside. As amyloid imaging becomes more widely applied, “it will reveal a lot of non-AD dementia,” said Cliff Jack of the Mayo Clinic, Rochester, Minnesota. In this sense, an amyloid-based diagnostic tool may, in effect, bend Alzheimer’s case estimates downward a bit, and in its wake give added prominence to a collection of other dementias that have tended to languish in AD’s shadow in terms of awareness, research attention, and funding.—Gabrielle Strobel.
This is Part 8 of a nine-part series. See also Part 1, Part 2, Part 3, Part 4, Part 5, Part 6, Part 7, Part 9. Download a PDF of the entire series.
No Available Comments
As if to ensure that nobody would leave early, the 6th Human Amyloid Imaging Conference, held on 12-13 January 2012 in Miami, Florida, held until the very end the agenda item that had inspired the most advance speculation and curiosity. The last two talks of the Conference were about efforts to fill a gaping void in AD imaging, that is, to develop a PET agent that will visualize the other pathologic lesion as defined by Alois Alzheimer. “A tau imaging agent might provide the missing link between Aβ pathology and neurodegeneration,” said Victor Villemagne of the University of Melbourne, Australia. The Conference up to this point had highlighted how visualizing but one of a number of proteins that together wreak havoc in the aging brain gets the field only so far (see Part 8 of this series).
Why have tau PET ligands been more elusive than Aβ ligands? It is not for lack of trying, scientists said. Tau is a difficult target for a tracer to find because its concentration in the brain is manifold lower than that of Aβ, and it is primarily intracellular. What’s worse, its many isoforms, post-translational modifications, and conformations make it quite the dizzying target. At HAI, Villemagne and Hartmut Kolb of Siemens Inc., in Culver City, California, each introduced a candidate tau ligand. One appears to offer proof of principle in humans, though less impressively than did PIB, and the other is a preclinical alternative poised to show its mettle in first human experiments this spring. First, the more advanced agent.
Villemagne, for the first time, showed human PET data of the tau radioligand 18F THK523. Scientists at Tohoku University in Sendai, Japan, have been developing THK523 since 2002 and, in collaboration with Villemagne’s group, recently reported that it binds tau selectively in tissue sections (Fodero-Tavoletti et al., 2011). The human volunteers presented at HAI constitute the first eight enrollees of a study calling for 35 people in all. Three have moderate Alzheimer’s; three have semantic dementia, a form of FTLD devoid of amyloid β and tau deposition; and two were cognitively normal elderly controls. To see where in the brain and how strongly THK523 binds compared to PIB, each person got an injection for both tracers.
How did the tau ligand do? In a word, so-so. As desired, it enters the brain well and washes out quickly, and it shows no difference between AD and normal in the cerebellum, a control region for AD pathology. In cortical regions known to be laden with neurofibrillary pathology in AD, THK523 did have differential retention between AD and controls, but the signal was disappointingly small, Villemagne said.
“Visually, you cannot see much of a difference,” Villemagne said, and visual inspection is important for eventual success in the clinic. Using quantitative analysis, the researchers do find higher THK523 retention in AD than in semantic dementia and controls. Among the controls, the 85-year-old showed more retention in the hippocampus than did the 70-year-old, which fits the age-related increase in neurofibrillary tangles there, Villemagne said. Also encouraging was that PIB and THK523 had different regional patterns of retention, where the latter follows the pattern of FDG hypometabolism, which is believed to reflect neurodegeneration downstream of amyloid deposition. In toto, then, this compound is a start, Villemagne, said, but not good enough (Villemagne et al., 2012).
In discussion, other scientists were more upbeat. Some pointed out that the signal THK523 yields is about the same or slightly better than what is achievable with PK11195, a PET ligand for neuroinflammation. Others pointed out that it does have higher affinity for tau and better selectivity than does FDDNP, another tracer that finds occasional use (Harada et al., SfN 2011). “You call THK523 a failure,” said Chet Mathis, a co-developer of PIB, “but it is a failure only by the high standards of PIB.” In any event, THK523 is not the last word. A second-generation compound by the same Japanese group, called 18F CPDE, is nearing first human tests as well; it is going to be developed commercially, Villemagne said.
Yet another tau tracer entered the stage at HAI, when Kolb, a nuclear medicine chemist who formerly worked in cancer and hypoxia, introduced the preclinical agents 18F-T807 and 18F-T808. These came out of competitive compound screens run on human brain slices. Rather than using human tau expressed in transgenic mice, Kolb decided to search for compounds that bind native tau as it occurs in patients. The binding affinity of the two tau ligands are 15 and 22 nM, respectively—perhaps a tad low, according to other scientists at HAI. Tied to fluorescent probes, the two compounds visualize human neurofibrillary tangles, Kolb said, adding that they bind tau with a 29-fold selectivity over Aβ and have shown no significant off-target binding. The 18F compounds stained tau pathology in autoradiographs of slices of 37 human brains, corresponding well to tau immunofluorescence, Kolb said. The compounds penetrate the blood-brain barrier in mice and primates, wash out within half an hour, and have shown no toxicity, he added (Kolb et al., 2012). Siemens has filed an exploratory IND for both compounds in December 2011, and intends to begin human testing this spring, Kolb said. There are no published data on these compounds.—Gabrielle Strobel.
This is Part 9 of a nine-part series. See also Part 1, Part 2, Part 3, Part 4, Part 5, Part 6, Part 7, Part 8. Download a PDF of the entire series.
No Available Comments
Comments
No Available Comments
Make a Comment
To make a comment you must login or register.