Biomarker Study in Down’s Syndrome Population May Yield Clues to AD
Quick Links
Longitudinal biomarker studies of Alzheimer’s disease have greatly advanced researchers’ understanding of disease progression. Now another such initiative seeks to further clarify the biological changes in early disease. The National Institutes of Health has committed $37 million to two five-year studies of Alzheimer’s disease biomarkers in people with Down’s syndrome. These individuals inherit an extra copy of the amyloid precursor protein gene and develop amyloid pathology by middle age. Thus, young and middle-aged adults with Down’s represent one of the largest genetically determined preclinical AD populations in the world. By charting their biomarker changes over time, researchers hope to identify some of the earliest signs of Alzheimer’s and perhaps develop new screening methods or diagnostic tests for the disease. They also hope the findings will enable preventive trials of new AD therapeutics in people with Down’s, said Laurie Ryan of the National Institute on Aging. NIA and the National Institute of Child Health and Human Development (NICHD) will jointly fund the new initiative.
Ryan said the initiative will be modeled on previous large biomarker studies such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). As with ADNI, all data from the new studies will be made freely available to researchers.
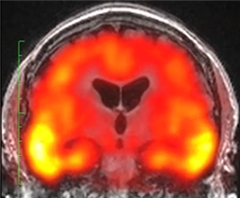
Tau Deposits Mirror AD.
Tau (yellow) accumulates in the medial temporal lobe of middle-aged people with Down’s syndrome in the same distinctive “batwing” pattern seen in prodromal Alzheimer’s. [Courtesy of Michael Rafii.]
Until recently, most people with Down’s syndrome did not live to old age. However, thanks to better health care, their average lifespan has doubled in the last 30 years, according to the National Down Syndrome Society. As a result, preventing or treating dementia in this group has become an urgent need. An estimated 90 percent of people with Down’s syndrome will develop dementia by the age of 70, said Nicole Schupf at Columbia University, New York. She and investigators at the New York State Institute for Basic Research in Developmental Disabilities and at the Kennedy Krieger Institute in Baltimore have been running an aging study in this population for years, and will lead part of the new NIH initiative.
Numerous groups around the world focus on aging in DS. The London Down Syndrome Consortium (LonDownS) in the United Kingdom runs a large study of how cognition changes with age and how genes contribute to the process. Researchers in the Trisomy 21 Research Society investigate ways to improve cognitive function across the lifespan (see Nov 2015 conference news). However, few if any groups have undertaken a systematic study of Alzheimer’s neuroimaging and fluid biomarkers. “I believe this is the largest, most comprehensive effort to do this type of analysis for individuals with Down’s syndrome,” said Melissa Parisi of the NICHD.
To achieve this goal, the NIH selected two multicenter research teams who will study distinct cohorts and focus on slightly different, but overlapping, biomarkers. Led by Benjamin Handen at the University of Pittsburgh, in collaboration with Brad Christian at the University of Wisconsin-Madison, Marwan Sabbagh at Barrow Neurological Institute, Phoenix, and Shahid Zaman at Cambridge University, U.K., one team will emphasize neuroimaging and cerebrospinal fluid biomarkers. Many of the researchers come from an Alzheimer’s research background. The other team, led by Schupf along with Ira Lott at the University of California, Irvine, and Wayne Silverman at the Kennedy Krieger Institute, will concentrate on blood-based proteomics and lipidomics. Additional clinical sites for this study will be led by Sharon Krinsky-McHale at the New York State Institute for Basic Research and Florence Lai and Diana Rosas at Massachusetts General Hospital, Boston. This team brings expertise in Down’s syndrome studies.
The Pittsburgh team has dubbed their study Neuroimaging in Aging Down Syndrome. The acronym, NIAD, purposely reuses the same letters as ADNI and DIAN to indicate its close relationship to those projects, said William Klunk at the University of Pittsburgh. All NIAD participants will undergo two amyloid PET scans with PiB at 32-month intervals, and two tau PET scans with the tracer T807/AV1451, also at the same interval. Each will have one FDG PET scan to look at brain glucose metabolism. The researchers hope to persuade at least half the participants to donate cerebrospinal fluid for analysis of Aβ42, total tau, and phosphorylated tau, Klunk said. NIAD will recruit 180 adults with Down’s and 40 controls without the condition. “We felt it was important to have some control biomarker data to compare against baseline values and longitudinal aging changes in people with Down’s syndrome,” Klunk noted. NIAD will enroll people 25 and older, with most participants between the ages of 35 and 55.
The Columbia study will skew slightly older. Schupf said they plan to enroll 280 participants with Down’s syndrome age 40 and up. “Between the two cohorts, we’ll have a wide age range to identify when biomarkers first start to change,” Schupf noted. The researchers will perform two amyloid PET scans with florbetapir 32 months apart on 45 participants without dementia, and one amyloid PET scan on an additional 45 participants with dementia. This design allows the researchers to detect changes in amyloid levels as initially non-demented volunteers develop MCI or AD, and contrast amyloid levels in people with established dementia from those in people without dementia. A subset of 45 people will be asked to donate cerebrospinal fluid.
Both teams have been conducting longitudinal biomarker studies on people with Down’s for years, and many of the current volunteers are expected to join the new studies. This means researchers will have run-in data for some participants. This includes some amyloid PET scans from the Pittsburgh group (see Hartley et al., 2014; Lao et al., 2015). The initiative will make use of the Down’s syndrome registry DS-Connect to recruit volunteers, Parisi said. Maintained by NIH, this registry helps connect people with Down’s syndrome to clinical studies that might interest them.
Beyond these differences in biomarker collection, the two teams will follow similar strategies. Both cohorts will undergo structural and functional MRIs and donate blood. They will sit for the same batteries of cognitive and neuropsychological tests. The researchers are harmonizing protocols so they can combine their data. Where possible, the new initiative will use the same protocols as DIAN and ADNI to facilitate comparisons across these studies, as well. To minimize noise in the data, all CSF samples will be analyzed by Anne Fagan at Washington University in St. Louis, Missouri. Sid O’Bryant at the University of North Texas Health Sciences Center, Fort Worth, will perform proteomics analyses on blood samples from both cohorts, and Gilbert Di Paolo and Robin Chan at Columbia will run all lipidomic assays, Schupf said.
In addition, the researchers will perform a GWAS using DNA from the combined cohort of 500 participants to look for risk or protective factors that modify the age of onset of AD. Schupf noted that, as in sporadic AD, people with Down’s develop dementia across a wide range of ages, and some of them escape it altogether. “That suggests there are additional genetic, biological, and environmental factors that may be important modifiers of risk and can accelerate or slow disease progression,” Schupf said. Because chromosome 21 contains genes involved in inflammation, studying a Down’s population may shed light on the role of the immune system in AD. To investigate how these factors may influence progression, Vitaly Vasilevko at UC Irvine will analyze a panel of cytokines from CSF samples, Fagan noted.
Researchers hope that besides revealing new biomarkers and risk factors, the findings will facilitate treatment trials to delay AD in people with Down’s syndrome. Amyloid-based treatments might work in this group because their disease results from excess production of normal amyloid precursor protein. “You could argue this is the most definitely amyloid-based form of Alzheimer’s disease,” Klunk said. In addition, Schupf noted that because people with Down’s rarely get heart disease, stroke, or hypertension, cardiovascular factors may contribute less to dementia in this group than in the general population. This might result in a purer form of AD.
Michael Rafii at the University of California, San Diego, called the new initiative a win-win because it will not only benefit people with Down’s, but could also help illuminate the timing of biomarker changes in early AD. Rafii is not involved in the initiative. However, at the 8th Clinical Trials in Alzheimer’s Disease conference in Barcelona, Spain, November 5-7, he presented baseline data from a three-year pilot study of Alzheimer’s biomarkers in 12 middle-aged people with Down’s (see Dec 2012 news; Rafii et al., 2015).
In this cohort, amyloid load was higher at older ages, and correlated with less hippocampal volume and worse performance on a memory test, Rafii said. As in sporadic AD, ApoE4 carriers accumulated the most amyloid. Tau pathology was also more widespread at older ages, expanding from the entorhinal cortex through the medial temporal lobe in the same distinctive “batwing” pattern recently reported for sporadic AD (see image above; Johnson et al., 2015). On these measures, the Down’s syndrome participants resembled patients with preclinical or prodromal AD.
By contrast, FDG PET imaging revealed both similarities and differences with the AD population. The Down’s syndrome participants had hypometabolism in the cingulate gyrus that resembled that seen in ADNI volunteers with AD. However, the Down’s syndrome cohort also had hypermetabolism in the frontal cortex, which is not seen in AD. This PET signal appears to be unique to the DS population. “We are trying to understand what that means functionally,” Rafii said. He has now collected data from the one- and two-year follow-up visits, which he hopes to publish soon. Overall, the findings from his pilot study suggest it will be feasible to follow AD biomarker progression in people with DS, Rafii noted.—Madolyn Bowman Rogers
References
News Citations
- Might Normalizing Brain Development Help in Down’s Syndrome?
- Natural History-Cum-Trials Initiative, Grants to Boost Down’s Research
Paper Citations
- Hartley SL, Handen BL, Devenny DA, Hardison R, Mihaila I, Price JC, Cohen AD, Klunk WE, Mailick MR, Johnson SC, Christian BT. Cognitive functioning in relation to brain amyloid-β in healthy adults with Down syndrome. Brain. 2014 Sep;137(Pt 9):2556-63. Epub 2014 Jul 2 PubMed.
- Lao PJ, Betthauser TJ, Hillmer AT, Price JC, Klunk WE, Mihaila I, Higgins AT, Bulova PD, Hartley SL, Hardison R, Tumuluru RV, Murali D, Mathis CA, Cohen AD, Barnhart TE, Devenny DA, Mailick MR, Johnson SC, Handen BL, Christian BT. The effects of normal aging on amyloid-β deposition in nondemented adults with Down syndrome as imaged by carbon 11-labeled Pittsburgh compound B. Alzheimers Dement. 2016 Apr;12(4):380-90. Epub 2015 Jun 13 PubMed.
- Rafii MS, Wishnek H, Brewer JB, Donohue MC, Ness S, Mobley WC, Aisen PS, Rissman RA. The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer's disease biomarkers in down syndrome. Front Behav Neurosci. 2015;9:239. Epub 2015 Sep 14 PubMed.
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016 Jan;79(1):110-9. Epub 2015 Dec 15 PubMed.
External Citations
Further Reading
News
- San Diego: Too Much APP Blocks Transport, Starves Down's Neurons
- Trisomy Trouble: Neurotrophin Signaling Defective in Down Syndrome
- New Findings Offer Hope for Down Syndrome
- Research Brief: Imaging Shows AD Pathology in Down’s Syndrome Brains
- Down’s Syndrome Loosens Regulation of the Entire Genome
- About-Turn—In Mouse Model of Down’s Syndrome, Inhibitory Neurotransmitter Excites
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.