Light SPARKs Neuronal Action Potentials
Quick Links
Imagine being able to turn on and off an individual neuron remotely—without prodding it with electrodes or challenging it with chemicals. Sound like a neuroscientist's dream? Well, maybe we need to be pinched, because in the November Nature Neuroscience, Dirk Trauner, Richard Kramer and colleagues at the University of California, Berkeley, reveal a technique for doing just that. The trick is to give neurons the right SPARK—a flash of light.
SPARK, short for synthetic photoisomerizable azobenzene-regulated K+ channel, is a voltage-gated potassium channel regulated by a light-sensitive gatekeeper. If you shine short wavelength light (~380 nm), the keeper opens the channel. Flash long wavelength light on the keeper and it shuts the channel down.
SPARKs were developed by joint first authors Matthew Banghart and Katherine Borges, and colleagues, who took advantage of a well-known property of voltage-gated potassium channels—they can be blocked by quaternary ammonium ions. With this in mind, Banghart and Borges synthesized a quaternary ammonium compound with a difference. Having the ammonium ion at one end, a maleimide group at the other, and an azo group in between, the compound, dubbed MAL-AZO-QA, can be switched from a cis to a trans configuration around the azo bond, the switch, of course, being light.
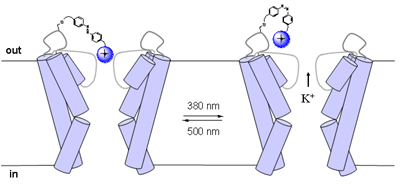
In the trans configuration, the molecule extends for about 17 Angstroms. In cis, it is only about 10 Angstroms long. This seven-Angstrom difference is critical because it is well-known that quaternary ammonium groups tethered to the lining of the channel must be just the right length to block the pore and, hence, the flow of potassium (see figure). The idea of the cis/trans light switch is to flip the ammonium ion in and out of this sweet spot.
Light-switchable potassium channel
Photoisomerization of an azo bond facilitates the opening and closing of a potassium channel. Long wavelength light converts the bond to the trans formation (left) stretching the maleimide-aso-quaternary ammonium ion compound so that the ammonium blocks the channel pore. Short wavelength light (right) flips the azo bond into the cis configuration, shortening the backbone of the compound and moving the ammonium ion away from the pore, allowing potassium ions to flow through. [Image courtesy of Dirk Trauner, Richard Kramer and Nature Neuroscience.]
To test the gatekeeper, the authors took advantage of the maleimide group. Because maleimide binds covalently to thiols, Banghart and Borges knew it would tether MAL-AZO-QA to a cysteine residue that they substituted for glutamine 422, which, it turns out, lies about 17 Angstroms from the pore of the voltage-gated channel. When they incubated the compound with Xenopus oocytes expressing the cys422 channel, the gatekeeper blocked conductance. When the authors shone ultraviolet light on the oocytes, current was restored, and by alternating ultraviolet with visible light, the authors could get the channel to close and open.
To test the light switch in a more physiological setting, the authors expressed multiply mutated voltage-gated K+ channels in hippocampal neurons (the channel was mutated to eliminate slow and fast inactivation, and to shift the voltage-dependent activation to hyperpolarized potentials, all of which result in high resting conductance in the context of the hippocampal neurons). When the authors incubated these neurons with MAL-AZO-QA and exposed them to ultraviolet light, action potentials were silenced within seconds. Visible light (500 nm) restored activity just as quickly.
“The power of this technique lies in its spatial and temporal accuracy, its noninvasiveness and its reversibility,” state the authors. SPARK channels may be used to control neuronal populations, individual neurons, or even parts of neurons, they add. They also propose that ligand-gated channels could be controlled in a similar manner, if the ligand binding site is a precise distance from a covalent attachment point on the protein.—Tom Fagan
References
No Available References
Further Reading
Primary Papers
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004 Dec;7(12):1381-6. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.