What Exactly Does the DJ Do?
Quick Links
Tao et al. and Honbou et al. recently reported the crystal structure of DJ-1 at resolutions of 1.8 and 1.95 Ångstroms, respectively (see ARF related news story), but the latest report by Greg Petsko and colleagues in the July 10 PNAS Early Edition online, tops that by almost another Ångstrom.
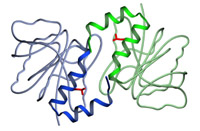
Model of the structure of the DJ dimer showing the side chain of lysine 166 (red). In PARK 7, the lysine is mutated to proline.
First author Mark Wilson reveals that the three independent determinations of the DJ-1 dimer differ very little, and all three labs are in agreement that the PARK 7 mutation, which results in a substitution of proline at position 166 for lysine (see model), causes a conformational bend in one of the helices that prevents the formation of the dimer. In fact, a paper just out from Mark Cookson's lab (Miller et al, 2003) indicates that the mutant DJ-1 is unstable and ends up in the proteasome, where it is degraded, and leads to a loss of DJ-1 function in the cell.
Where the three labs differ, suggests Wilson, is in predicting just what DJ-1 does. Based on homology to other proteins, Tao and Tong suggest that it may be a caspase-like protease (see ARF related news story), but as Wilson and colleagues point out, DJ-1 homologs also include kinases and amidotransferases, and these authors favor a nonproteolytic role for DJ-1, perhaps in the oxidative response. It will be fascinating to see who has made the right call.-Tom Fagan.
References: Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA. The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease. Proc Natl Acad Sci U S A. 2003 Aug 5 ; 100(16):9256-61. Abstract
References
News Citations
Paper Citations
- Miller DW, Ahmad R, Hague S, Baptista MJ, Canet-Aviles R, McLendon C, Carter DM, Zhu PP, Stadler J, Chandran J, Klinefelter GR, Blackstone C, Cookson MR. L166P mutant DJ-1, causative for recessive Parkinson's disease, is degraded through the ubiquitin-proteasome system. J Biol Chem. 2003 Sep 19;278(38):36588-95. PubMed.
Other Citations
Further Reading
Papers
- Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA. The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9256-61. PubMed.
Primary Papers
- Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA. The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9256-61. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.