Past Webinar
Stress Granules and Neurodegenerative Disease—What’s the Scoop?
Quick Links
Introduction
The formation of cytoplasmic inclusions called stress granules is one of nature’s many ways of coping with adverse situations. It appears that these inclusions sequester non-essential messenger RNAs, allowing cells to focus on making proteins, such as chaperones, that protect against a variety of stressful insults. Recent work suggests that stress granules may figure in the pathogenesis of amyotrophic lateral sclerosis and frontotemporal lobar degeneration, where stress granule markers decorate pathological protein aggregates in affected neurons. What do stress granules do in disease? Are they part of the problem or part of the solution? What about AD?
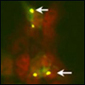 TDP-43 in stress granules |
Image caption: In human neuroblastoma cells transfected with TDP-43, inclusions (arrows) test positive for both TDP-43 (green) and the stress granule marker TIA-1 (red). View larger image |
This Webinar on stress granules and their relevance to neurodegeneration was led by Ben Wolozin, Boston University; Daryl Bosco, University of Massachusetts Medical Center, Worcester; and Dorothee Dormann, Ludwig-Maximilians-University, Munich, Germany. Aaron Gitler, University of Pennsylvania, Philadelphia, joined them for a panel discussion.
Media
Background
Background Text
Tom Fagan
Never heard of stress granules? Not to worry; they are new to the neurodegeneration scene, and that is why we are featuring them in this discussion. Stress granules are cytoplasmic inclusions of proteins that play key roles in translation of messenger RNA. They form naturally when a cell deals with stress, such as heat shock, oxygen deprivation, or oxidative damage. They can also be experimentally induced by knocking down (Mokas et al., 2009) or inhibiting (Kedersha et al., 1999) key translation factors, or by overexpressing proteins or adding drugs that block translation (Dang et al., 2006; Mazroui et al., 2006). The precise role of stress granules is unclear, but one theory is that they sequester mRNA transcripts that are not used in coping with stress (see Anderson and Kedersha, 2008). Stress granules dissipate when the stress has passed, and their disassembly correlates with the resumption of general protein synthesis (Mazroui et al., 2007).
These granules contain many of the proteins that are involved in translation, including ribosomal subunits and translational initiation factors such as eukaryotic initiation factors 2, 3, and 4. They also contain polyA mRNA and polyA binding proteins (for a review, see Anderson and Kedersha, 2006) and other components that seem to recruit mRNA to the granules, such as T cell intracellular antigen 1 (TIA-1) and RasGAP-associated endoribonuclease (G3BP). Proteins implicated in neurodegenerative or neurologic diseases have also been linked to these bodies. Ataxin-2, which causes a form of spinocerebellar ataxia when mutated, and survival motor neuron (SMN), the protein at the root of spinal muscular atrophy, both disrupt stress granule formation (Nonhoff et al., 2007; Hua and Zhou, 2004). SMN binds to stress granule proteins, as does huntingtin (Waelter et al., 2001) and prion protein (Goggin et al., 2008). There also seems to be a link between stress granules and the fragile X mental retardation protein (FMRP), whose normal role is to repress translation of RNA in dendrites. FMRP associates with RNAs and proteins found in stress granules (Vanderklish and Edelman, 2005; Linder et al., 2008), and mutations in FMRP protein that cause fragile X syndrome also disrupt these inclusions (Didiot et al., 2008).
More recently, researchers linked stress granules to amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration. Wolozin and colleagues reported that the RNA-binding protein TDP-43 gets incorporated into stress granules (see ARF related news story on Liu-Yesucevitz et al., 2010). TDP-43 mutations have emerged as a cause of a small subset of familial ALS cases and some cases of frontotemporal dementia, and aggregates of the protein occur in a majority of ALS cases studied on autopsy. TDP-43 appears to interact with TIA-1, both directly and via RNA. In postmortem tissue from people with ALS and FTLD-U, TDP-43 also colocalizes in inclusions containing TIA-1 and eukaryotic initiation factor 3, suggesting that the TDP-43 inclusions formed in these diseases are stress granules. Interestingly, Gitler and colleagues reported that ataxin-2, normally associated with ataxia, increases a person’s risk for ALS when it undergoes a moderate polyglutamate expansion (Elden et al., 2010). Since ataxin-2 binds stress granules, this strengthens the link between those inclusions and ALS.
Further support for the involvement of these granules in ALS comes from Bosco’s lab and also from Christian Haass’ lab at Ludwig-Maximilians-University, Munich, Germany. Both found that mutants of another RNA binding protein that causes familial ALS, fused in sarcoma (FUS), also end up in stress granules (Dorman et al., 2010 and Bosco et al., 2010). Bosco’s group in collaboration with Larry Hayward, also at UMass Medical Center, found that in cell and zebrafish models of ALS, FUS is more likely to end up in these granules when the scientists apply additional stress, for example, heat shock or oxidative damage.
How do these stress granules fit in with the pathology of ALS/FTLD? Do mutants of TDP-43 and FUS simply make cells more vulnerable to stress, or is there more to the story? What about other neurodegenerative diseases, for example, Alzheimer’s?
References
News Citations
Paper Citations
- Mokas S, Mills JR, Garreau C, Fournier MJ, Robert F, Arya P, Kaufman RJ, Pelletier J, Mazroui R. Uncoupling stress granule assembly and translation initiation inhibition. Mol Biol Cell. 2009 Jun;20(11):2673-83. PubMed.
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999 Dec 27;147(7):1431-42. PubMed.
- Dang Y, Kedersha N, Low WK, Romo D, Gorospe M, Kaufman R, Anderson P, Liu JO. Eukaryotic initiation factor 2alpha-independent pathway of stress granule induction by the natural product pateamine A. J Biol Chem. 2006 Oct 27;281(43):32870-8. PubMed.
- Mazroui R, Sukarieh R, Bordeleau ME, Kaufman RJ, Northcote P, Tanaka J, Gallouzi I, Pelletier J. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation. Mol Biol Cell. 2006 Oct;17(10):4212-9. PubMed.
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008 Mar;33(3):141-50. PubMed.
- Mazroui R, Di Marco S, Kaufman RJ, Gallouzi IE. Inhibition of the ubiquitin-proteasome system induces stress granule formation. Mol Biol Cell. 2007 Jul;18(7):2603-18. PubMed.
- Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006 Mar 13;172(6):803-8. PubMed.
- Nonhoff U, Ralser M, Welzel F, Piccini I, Balzereit D, Yaspo ML, Lehrach H, Krobitsch S. Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol Biol Cell. 2007 Apr;18(4):1385-96. PubMed.
- Hua Y, Zhou J. Survival motor neuron protein facilitates assembly of stress granules. FEBS Lett. 2004 Aug 13;572(1-3):69-74. PubMed.
- Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell. 2001 May;12(5):1393-407. PubMed.
- Goggin K, Beaudoin S, Grenier C, Brown AA, Roucou X. Prion protein aggresomes are poly(A)+ ribonucleoprotein complexes that induce a PKR-mediated deficient cell stress response. Biochim Biophys Acta. 2008 Mar;1783(3):479-91. PubMed.
- Vanderklish PW, Edelman GM. Differential translation and fragile X syndrome. Genes Brain Behav. 2005 Aug;4(6):360-84. PubMed.
- Linder B, Plöttner O, Kroiss M, Hartmann E, Laggerbauer B, Meister G, Keidel E, Fischer U. Tdrd3 is a novel stress granule-associated protein interacting with the Fragile-X syndrome protein FMRP. Hum Mol Genet. 2008 Oct 15;17(20):3236-46. PubMed.
- Didiot MC, Subramanian M, Flatter E, Mandel JL, Moine H. Cells lacking the fragile X mental retardation protein (FMRP) have normal RISC activity but exhibit altered stress granule assembly. Mol Biol Cell. 2009 Jan;20(1):428-37. PubMed.
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Vanderwyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, Petrucelli L, Wolozin B. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010;5(10):e13250. PubMed.
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rüb U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM, Gitler AD. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010 Aug 26;466(7310):1069-75. PubMed.
- Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Schmid B, Neumann M, Haass C. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010 Aug 18;29(16):2841-57. PubMed.
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ Jr, Sapp P, McKenna-Yasek D, Brown RH Jr, Hayward LJ. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010 Nov 1;19(21):4160-75. Epub 2010 Aug 10 PubMed.
Other Citations
Further Reading
Papers
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci. 2000 Jun 15;20(12):4389-97. PubMed.
- Piacentini R, Civitelli L, Ripoli C, Marcocci ME, De Chiara G, Garaci E, Azzena GB, Palamara AT, Grassi C. HSV-1 promotes Ca2+ -mediated APP phosphorylation and Aβ accumulation in rat cortical neurons. Neurobiol Aging. 2011 Dec;32(12):2323.e13-26. Epub 2010 Jul 31 PubMed.
Panelists
-
 Benjamin Wolozin, M.D., Ph.D.
Boston University School of Medicine
Benjamin Wolozin, M.D., Ph.D.
Boston University School of Medicine
-
 Daryl Bosco, Ph.D.
University of Massachusetts Medical Center
Daryl Bosco, Ph.D.
University of Massachusetts Medical Center
-
 Aaron Gitler, Ph.D.
Stanford University
Aaron Gitler, Ph.D.
Stanford University
-
 Dorothee Dormann, Ph.D.
JGU Mainz
Dorothee Dormann, Ph.D.
JGU Mainz

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.