Confirmed: Women Accumulate Tangles Faster Than Do Men
Quick Links
Numerous studies have hinted that women are particularly susceptible to tau pathology, but scientists have lacked definitive data. Now, a meta-analysis of six longitudinal tau PET studies offers the strongest evidence yet. In the March 3 JAMA Neurology, scientists led by Rachel Buckley at Massachusetts General Hospital, Charlestown, reported that among people with amyloid plaques, tangles in several brain regions built up faster in women than in men. Carrying an APOE4 allele further egged on tangle spread in women, independent of its effect of amyloid.
- Among people with brain amyloid, tangles built up faster in women than in men.
- APOE4 also accelerated tangle buildup in women, but not men.
- Women might need to start amyloid immunotherapy sooner.
The findings imply that women might need to take anti-amyloid therapies at earlier disease stages, though it is unclear exactly when women should start treatment, or what biological factors underlie this sex difference, Buckley told Alzforum.
Nicolai Franzmeier and Davina Biel at the Ludwig Maximilians University, Munich, called the data robust and convincing. Pierre Tariot at the Banner Alzheimer Institute, Phoenix, agreed. “The authors make a compelling case that women experience more aggressive tau accumulation than men,” Tariot wrote to Alzforum (comments below).
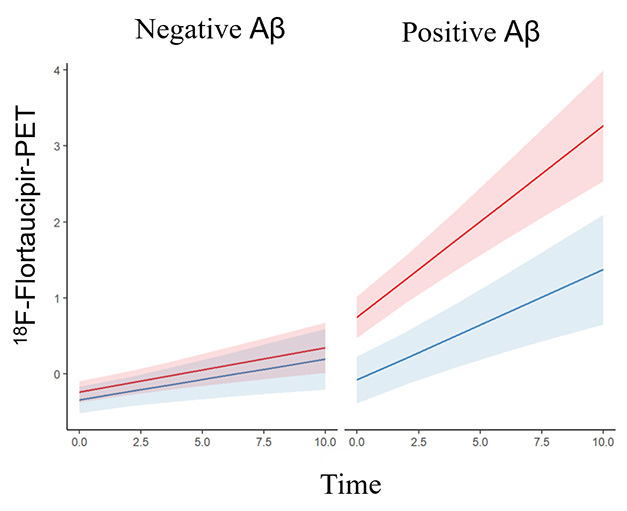
Not the Same. In amyloid-negative people (left) few tangles accumulate over a decade. In amyloid-positives (right), tangles grow faster in women (orange) than men (blue). [Courtesy of Coughlan et al., American Medical Association, 2025.]
Most previous studies reporting higher tangle loads in women were cross-sectional (Feb 2019 news; Buckley et al., 2020; Edwards et al., 2021). This left unanswered whether there was a difference in accumulation, or if women were more resilient to tangles and thus tolerated higher loads at a given clinical stage. A few longitudinal tau PET studies strengthened the case for the former, but these had fewer than 500 participants, and in some cases fell short of statistical significance (Smith et al., 2020; Jack et al., 2020; Wang et al., 2024).
To get a stronger answer, first author Gillian Coughlan analyzed data from six longitudinal tau PET studies: the Alzheimer’s Disease Neuroimaging Initiative, the Berkeley Aging Cohort Study, BioFINDER 1, the Harvard Aging Brain Study, the Mayo Clinic Study of Aging, and the Wisconsin Registry for Alzheimer Prevention. Altogether, the meta-analysis comprised 1,376 participants who were cognitively healthy at baseline. They were 72 years old on average, and 55 percent were women. All had a baseline amyloid scan as well as multiple tau PET scans over an average three-year time span. Five studies used the tau tracer flortaucipir, while WRAP used MK-6240.
In the full cohort, tangle accumulation did not vary by sex. Most people in this cognitively healthy cohort were amyloid-negative and had little to no change in tau PET signal over the course of the study. Among the 401 participants who were amyloid-positive at baseline, however, it was a different story. Women accumulated tangles faster than men in the inferior temporal lobe, the temporal fusiform gyri, and the lateral occipital cortex. “[These regions] serve as key entry points for the widespread propagation of tau across the brain,” Franzmeier and Biel noted.
In the five studies that used flortaucipir, the effect was small, with women adding 0.001 to 0.020 SUVR more than men in these regions per year. In WRAP, the effect was larger, varying from 0.037 SUVR in the temporal fusiform gyri to 0.074 in the lateral occipital cortex. MK-6240 is more sensitive than flortaucipir, with a larger dynamic range, suggesting that this tracer might be better able to pick up on small changes in tangle load.
On the other end of the scale, the one study that showed no sex differences was BACS. It had the lowest threshold for amyloid positivity at 10 centiloids, and the oldest participants, with an average baseline age of 76. Tangles are known to accumulate faster at higher amyloid loads and younger ages.
The authors also looked for a sex effect among APOE4 carriers. Here they found a subtle difference, with female E4 carriers accumulating more tangles in the inferior temporal lobe than did male carriers, even when amyloid load was taken into account. “That says to me that amyloid is not the only driver,” Buckley noted. Animal studies have shown that the E4 allele can worsen tau pathology independently of amyloid (Sep 2017 news; Oct 2019 news; Nov 2023 news). Another recent study reported a stronger correlation between the number of ApoE alleles and levels of total tau in the CSF, a marker of neurodegeneration, in women than in men (Xu et al., 2025).
Regarding what causes this sex difference, previous research implicates hormonal changes at menopause, or the effect of X-linked genes (Buckley et al., 2022; May 2023 news; Oct 2022 news). Buckley plans to pursue both possibilities.
What does this mean for women’s health? Faster tangle accumulation could blunt the efficacy of anti-amyloid immunotherapy for women. Trial data support this. In the gantenerumab Phase 3 studies, women had a higher baseline tangle load than did men, and gained no cognitive benefit from the medication (Apr 2023 conference news). In the lecanemab Phase 3 trial, men also reaped more benefit than women (Dec 2022 conference news). However, in donanemab Phase 3, where participants were selected by baseline tangle load, there was no sex difference (Jul 2023 conference news).
“The optimal threshold for initiating anti-Aβ immunotherapies may differ by sex, with earlier intervention in women potentially maximizing clinical benefit,” Franzmeier and Biel wrote to Alzforum. That said, Tariot cautioned that other explanations might account for the sex difference in trials, such as distinct co-pathologies or medication use in female versus male participants. Buckley agrees more research is needed before making treatment recommendations. She wants to know when tangle accumulation first diverges between women and men. In the meantime, Buckley believes scientists should consider stratifying baseline and outcome trial data by sex to help the field gather more data.—Madolyn Bowman Rogers
References
News Citations
- Is a Woman’s Brain More Susceptible to Tau Pathology?
- ApoE4 Makes All Things Tau Worse, From Beginning to End
- In Tauopathy, ApoE Destroys Neurons Via Microglia
- Do APOE4’s Lipid Shenanigans Trigger Tauopathy?
- Are Hormones to Blame for High Tau in Women?
- Ubiquitin Peptidase Linked to Increased Tau Pathology in Women
- What Happens After Amyloid Plaque Removal? Who Benefits Most?
- Dare We Say Consensus Achieved: Lecanemab Slows the Disease
- Donanemab Data Anchors Upbeat AAIC
Therapeutics Citations
Paper Citations
- Buckley RF, Scott MR, Jacobs HI, Schultz AP, Properzi MJ, Amariglio RE, Hohman TJ, Mayblyum DV, Rubinstein ZB, Manning L, Hanseeuw BJ, Mormino EC, Rentz DM, Johnson KA, Sperling RA. Sex Mediates Relationships Between Regional Tau Pathology and Cognitive Decline. Ann Neurol. 2020 Nov;88(5):921-932. Epub 2020 Aug 31 PubMed.
- Edwards L, La Joie R, Iaccarino L, Strom A, Baker SL, Casaletto KB, Cobigo Y, Grant H, Kim M, Kramer JH, Mellinger TJ, Pham J, Possin KL, Rosen HJ, Soleimani-Meigooni DN, Wolf A, Miller BL, Rabinovici GD. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer's continuum: greater tau-PET retention in females. Neurobiol Aging. 2021 Sep;105:86-98. Epub 2021 Apr 22 PubMed.
- Smith R, Strandberg O, Mattsson-Carlgren N, Leuzy A, Palmqvist S, Pontecorvo MJ, Devous MD, Ossenkoppele R, Hansson O. The accumulation rate of tau aggregates is higher in females and younger amyloid-positive subjects. Brain. 2020 Dec 1;143(12):3805-3815. PubMed.
- Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Botha H, Graff-Radford J, Jones DT, Ferman TJ, Boeve BF, Kantarci K, Vemuri P, Mielke MM, Whitwell J, Josephs K, Schwarz CG, Senjem ML, Gunter JL, Petersen RC. Predicting future rates of tau accumulation on PET. Brain. 2020 Oct 1;143(10):3136-3150. PubMed.
- Wang YT, Therriault J, Servaes S, Tissot C, Rahmouni N, Macedo AC, Fernandez-Arias J, Mathotaarachchi SS, Benedet AL, Stevenson J, Ashton NJ, Lussier FZ, Pascoal TA, Zetterberg H, Rajah MN, Blennow K, Gauthier S, Rosa-Neto P, Alzheimer’s Disease Neuroimaging Initiative. Sex-specific modulation of amyloid-β on tau phosphorylation underlies faster tangle accumulation in females. Brain. 2024 Apr 4;147(4):1497-1510. PubMed.
- Xu X, Kwon J, Yan R, Apio C, Song S, Heo G, Yang Q, Timsina J, Liu M, Budde J, Blennow K, Zetterberg H, Lleó A, Ruiz A, Molinuevo JL, Lee VM, Deming Y, Heslegrave AJ, Hohman TJ, Pastor P, Peskind ER, Albert MS, Morris JC, Park T, Cruchaga C, Sung YJ. Sex Differences in Apolipoprotein E and Alzheimer Disease Pathology Across Ancestries. JAMA Netw Open. 2025 Mar 3;8(3):e250562. PubMed.
- Buckley RF, O'Donnell A, McGrath ER, Jacobs HI, Lois C, Satizabal CL, Ghosh S, Rubinstein ZB, Murabito JM, Sperling RA, Johnson KA, Seshadri S, Beiser AS. Menopause Status Moderates Sex Differences in Tau Burden: A Framingham PET Study. Ann Neurol. 2022 Jul;92(1):11-22. Epub 2022 May 17 PubMed.
Further Reading
News
- ApoE and Tau: Unholy Alliance Spawns Neurodegeneration
- Squelching ApoE in Astrocytes of Tau-Ravaged Mice Dampens Degeneration
- Sans TREM2, ApoE4 Drives Microgliosis and Atrophy in Tauopathy Model
- Do Brain Changes at Menopause Make Women More Prone to Alzheimer’s?
- ApoE4 and Tau in Alzheimer’s: Worse Than We Thought? Especially in Women
- Women and Men Differ in Their Genetic Risk for Alzheimer’s Progression
Primary Papers
- Coughlan GT, Klinger HM, Boyle R, Betthauser TJ, Binette AP, Christenson L, Chadwick T, Hansson O, Harrison TM, Healy B, Jacobs HI, Hanseeuw B, Jonaitis E, Jack CR Jr, Johnson KA, Langhough RE, Properzi MJ, Rentz DM, Schultz AP, Smith R, Seto M, Johnson SC, Mielke MM, Shirzadi Z, Yau WW, Manson JE, Sperling RA, Vemuri P, Buckley RF, Alzheimer’s Disease Neuroimaging Initiative. Sex Differences in Longitudinal Tau-PET in Preclinical Alzheimer Disease: A Meta-Analysis. JAMA Neurol. 2025 Mar 3; Epub 2025 Mar 3 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Ludwig Maximilians University
Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany
This meta-analysis by Coughlan et al. provides robust evidence across six longitudinal cohorts, showing that women accumulate tau pathology faster than men in preclinical AD. This work is of high clinical relevance, as the transition from amyloidosis to tauopathy marks the critical tipping point for progression to mild cognitive impairment and dementia (Ossenkoppele et al., 2022) where tau drives neurodegeneration and cognitive deterioration (Biel et al., 2021; La Joie et al., 2020). The study shows convincingly that women at the stage of preclinical AD exhibit significantly greater tau-PET increases over time, particularly in brain regions known to serve as key entry points for the widespread propagation of tau across the brain (Lee et al., 2022). Therefore, these findings reinforce growing evidence that women are at heightened risk for tau pathology in the presence of Aβ, which may contribute to the higher incidence and prevalence of AD in females. Importantly, this study is the largest to date to demonstrate the strengthened effect of Aβ on tau in women longitudinally, expanding upon prior smaller-scale observations (Buckley et al., 2019; Smith et al., 2020; Wang et al., 2024).
Coughlan et al.’s results may have key implications for sex-specific treatment strategies in AD. If women accumulate tau pathology sooner after Aβ deposition, they may require earlier therapeutic intervention to prevent runaway tau spread. This is particularly urgent given that anti-Aβ therapies show diminished efficacy once tau pathology is established. Indeed, the donanemab Trailblazer-Alz 2 trial demonstrated that higher tau-PET levels at treatment initiation were a clear limiting factor for clinical efficacy, underscoring the need for precise timing of intervention prior to the onset of severe tauopathy to yield clinical benefit (Sims et al., 2023). In practical terms, the optimal threshold for initiating anti-Aβ immunotherapies may therefore differ by sex, with earlier intervention in women potentially maximizing clinical benefit.
Emerging trial data support this tailored approach. Post hoc analyses of the Phase 3 lecanemab trial suggest a somewhat attenuated clinical benefit in women versus men, potentially because a subset of women had already progressed further along the Aβ-tau axis at treatment initiation, reducing the impact of Aβ removal (van Dyck et al., 2023). Aligning treatment timing with individual pathology trajectories, in part by accounting for sex differences in Ab–tau dynamics, may therefore enhance therapeutic efficacy.
More broadly, these findings emphasize that biomarker-based stratification in AD should not rely on one-size-fits-all thresholds, as they may obscure biological differences between men and women. Moving toward precision medicine—incorporating sex as a modulator of the Ab-tau axis in trial design and clinical decision-making—could therefore optimize outcomes.
What remains open, however, are the biological drivers of these sex-specific differences in Aβ-driven tau accumulation. Previous studies have suggested that androgens may protect against tau hyperphosphorylation, putting men at lower risk of AD compared to women, in whom testosterone levels are typically lower than in men and further decreased after menopause (Papasozomenos et al., 2002; Papasozomenos, 1997; Sundermann et al., 2020). In addition, neuroinflammation may play a key role, where women show stronger neuroinflammation-related tau hyperphosphorylation than men (Biel et al., 2025), and stronger inflammatory-related tau deposition (Biechele et al., 2024).
Disentangling the biological mechanisms that underlie sex differences in Aβ-related tauopathy should be a focus of AD research to determine potentially novel treatment targets and address the increased prevalence of AD in women versus men.
References:
Ossenkoppele R, Pichet Binette A, Groot C, Smith R, Strandberg O, Palmqvist S, Stomrud E, Tideman P, Ohlsson T, Jögi J, Johnson K, Sperling R, Dore V, Masters CL, Rowe C, Visser D, van Berckel BN, van der Flier WM, Baker S, Jagust WJ, Wiste HJ, Petersen RC, Jack CR Jr, Hansson O. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med. 2022 Nov;28(11):2381-2387. Epub 2022 Nov 10 PubMed.
Biel D, Brendel M, Rubinski A, Buerger K, Janowitz D, Dichgans M, Franzmeier N, Alzheimer’s Disease Neuroimaging Initiative (ADNI). Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheimers Res Ther. 2021 Aug 12;13(1):137. PubMed.
La Joie R, Visani AV, Baker SL, Brown JA, Bourakova V, Cha J, Chaudhary K, Edwards L, Iaccarino L, Janabi M, Lesman-Segev OH, Miller ZA, Perry DC, O'Neil JP, Pham J, Rojas JC, Rosen HJ, Seeley WW, Tsai RM, Miller BL, Jagust WJ, Rabinovici GD. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020 Jan 1;12(524) PubMed.
Lee WJ, Brown JA, Kim HR, La Joie R, Cho H, Lyoo CH, Rabinovici GD, Seong JK, Seeley WW, Alzheimer’s Disease Neuroimaging Initiative. Regional Aβ-tau interactions promote onset and acceleration of Alzheimer's disease tau spreading. Neuron. 2022 Jun 15;110(12):1932-1943.e5. Epub 2022 Apr 19 PubMed.
Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HI, Papp KV, Amariglio RE, Properzi MJ, Schultz AP, Kirn D, Scott MR, Hedden T, Farrell M, Price J, Chhatwal J, Rentz DM, Villemagne VL, Johnson KA, Sperling RA. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured By Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol. 2019 Feb 4; PubMed.
Smith R, Strandberg O, Mattsson-Carlgren N, Leuzy A, Palmqvist S, Pontecorvo MJ, Devous MD, Ossenkoppele R, Hansson O. The accumulation rate of tau aggregates is higher in females and younger amyloid-positive subjects. Brain. 2020 Dec 1;143(12):3805-3815. PubMed.
Wang YT, Therriault J, Servaes S, Tissot C, Rahmouni N, Macedo AC, Fernandez-Arias J, Mathotaarachchi SS, Benedet AL, Stevenson J, Ashton NJ, Lussier FZ, Pascoal TA, Zetterberg H, Rajah MN, Blennow K, Gauthier S, Rosa-Neto P, Alzheimer’s Disease Neuroimaging Initiative. Sex-specific modulation of amyloid-β on tau phosphorylation underlies faster tangle accumulation in females. Brain. 2024 Apr 4;147(4):1497-1510. PubMed.
Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES, Collins EC, Solomon P, Salloway S, Apostolova LG, Hansson O, Ritchie C, Brooks DA, Mintun M, Skovronsky DM, TRAILBLAZER-ALZ 2 Investigators. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023 Aug 8;330(6):512-527. PubMed.
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer's Disease. N Engl J Med. 2023 Jan 5;388(1):9-21. Epub 2022 Nov 29 PubMed.
Papasozomenos SC, Shanavas A. Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer's disease. Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1140-5. PubMed.
Papasozomenos SC. The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: implications for Alzheimer disease. Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6612-7. PubMed.
Sundermann EE, Panizzon MS, Chen X, Andrews M, Galasko D, Banks SJ, Alzheimer’s Disease Neuroimaging Initiative. Sex differences in Alzheimer's-related Tau biomarkers and a mediating effect of testosterone. Biol Sex Differ. 2020 Jun 19;11(1):33. PubMed.
Biel D, Suárez-Calvet M, Dewenter A, Steward A, Roemer SN, Dehsarvi A, Zhu Z, Pescoller J, Frontzkowski L, Kreuzer A, Haass C, Schöll M, Brendel M, Franzmeier N. Female sex is linked to a stronger association between sTREM2 and CSF p-tau in Alzheimer's disease. EMBO Mol Med. 2025 Feb;17(2):235-248. Epub 2025 Jan 10 PubMed.
Biechele G, Rauchmann BS, Janowitz D, Buerger K, Franzmeier N, Weidinger E, Guersel S, Schuster S, Finze A, Harris S, Lindner S, Albert NL, Wetzel C, Rupprecht R, Rominger A, Palleis C, Katzdobler S, Burow L, Kurz C, Zaganjori M, Trappmann LK, Goldhardt O, Grimmer T, Haeckert J, Keeser D, Stoecklein S, Morenas-Rodriguez E, Bartenstein P, Levin J, Höglinger GU, Simons M, Perneczky R, Brendel M. Associations between sex, body mass index and the individual microglial response in Alzheimer's disease. J Neuroinflammation. 2024 Jan 23;21(1):30. PubMed.
Banner Alzheimer's Institute
This strikes me as a thoughtful and rigorous effort to improve our understanding of the apparent increased risk of having a diagnosis of AD in women than men, the reasons for which are not fully elucidated. The authors make a compelling case that women experience more aggressive tau accumulation than men.
I concur with their comments about the paper’s main limitations, chief among them that lack of racial, ethnic, education, and other diversity limits generalizability. The authors also note that there could be a sex-specific tau response to Aβ that is triggered by other, as-yet unknown determinants of risk, such as menopause, aberrant inflammation, or cardiovascular factors, as well as possible effects of psychosocial, and/or cultural gendered factors. We should add possible lifestyle effects to the list of unknowns.
Further caution is needed in extrapolating from these results to the as-yet unexplained possible sex differences in response to monoclonal antibody therapy. While beyond the scope of this comment, I would suggest that there is a great deal we do not know about this phenomenon. There could be myriad explanations, ranging from different concomitant medications to potential sex-related differential co-pathologies in the brains of the participants in treatment (and natural history) studies. These acknowledgements and limitations suggest we need to exercise caution in assuming we know enough to take definitive action on the important findings reported here.
UCSF
This paper is a massive accomplishment, representing the most robust evidence yet of female-specific vulnerability to tau aggregation. Applying a meta-analytic approach, the authors pooled estimates across six studies, including more than 1,300 participants in the largest longitudinal query into sex differences in AD biology to date. This was no easy feat. These data converge with existing studies to compellingly show that, put simply, females carry a higher AD-related tau burden. This sex difference is becoming indisputable. It has been shown across measurement tools—in blood, cerebrospinal fluid, brain tissue, and PET imaging—and has now extended to include longitudinal aggregation rates.
I agree with the authors that the question about timing is important. When does this start to happen for females? Coughlin et al. show an intercept (baseline) difference in tau burden in females across almost all cohorts evaluated. In fact, baseline tau at least in part mediated, i.e., explained, the sex difference in longitudinal tau aggregation. If we can identify the window during which tau takes off in females, this will provide foundational insight into AD pathogenesis, which is relevant for all brains.
Why and how is this happening? Do females harbor a different form of tau phosphorylation compared to males? These questions are important given that lecanemab shows less than half the efficacy in females compared to males.
One other interesting datapoint in this study is that despite carrying higher AD biologic burden, females performed better on cognitive testing compared to males. This is consistent with other studies showing that despite higher tau, females show better or comparable clinical performances than males during early stages of disease, an effect that appears to reverse after symptom onset, during which females decline more precipitously. These patterns are consistent with a “cognitive resilience” phenomenon in females. Together, it begs the question, can we leverage the female brain to unlock new therapeutic approaches for AD?
Make a Comment
To make a comment you must login or register.