Proteins in Biofluids Foreshadow Dementia by 30 Years
Quick Links
Decades before a person's first memory problems surface, changes happen in their biofluids that suggest their path toward dementia has already been forged. Two studies identified proteomic signals, in cerebrospinal fluid and in plasma, that may point to processes involved in the earliest stages of neurodegenerative disease known to date. One, published August 7 in Nature Medicine and led by Erik Johnson at Emory University in Atlanta, took stock of 59 proteins among hundreds of carriers of autosomal-dominant AD mutations. As early as 30 years prior to their estimated symptom onset, proteins of the extracellular matrix—including some known to mingle with Aβ plaques—started to rise. This was followed by sequential alterations in proteins involved in glucose metabolism, inflammation, and synaptic function as disease onset neared.
- CSF proteomics study finds extracellular matrix, metabolism, inflammatory, and synaptic proteins change decades before symptom onset in familial AD.
- In plasma, proteins involved in proteostasis, vascular function, and immunity predict 25-year dementia risk.
- Some, such as GDF15, are not detected in the brain. Do they influence dementia from the outside?
“This paper is a milestone, providing, for the first time, in-depth insights into when and which molecular processes are dysregulated in autosomal-dominant AD,” wrote Betty Tijms of Amsterdam University Medical Center and Pieter Jelle Visser of Maastricht University in the Netherlands (comment below).
The second study, led by Keenan Walker at the National Institute on Aging and Josef Coresh at Johns Hopkins University, both in Baltimore, took a different approach. They related the abundance of thousands of proteins in plasma samples collected in mid-life to their donors' risk of dementia more than 20 years later. Published July 19 in Science Translational Medicine, that study also identified extracellular matrix and inflammation as dementia-related processes, as well as protein trafficking and cell disposal pathways such as autophagy. Interestingly, some of the dementia-related proteins were not in the brain, suggesting that they may have influenced disease from the periphery.
Gerold Schmitt-Ulms, University of Toronto, called this work groundbreaking. “In addition to providing testable hypotheses by putting a spotlight on specific blood proteins […] the study adds to the concept that the earliest changes in the levels of blood proteins that may be helpful for dementia risk prediction can be obscured by the time the disease progresses to dementia.”
Johnson’s paper is the latest in a long line of proteomics research at Emory. Previously, the scientists surveyed proteins in postmortem brain samples from people who had died at different stages of sporadic, late-onset AD. They detected 40 groups of proteins that correlated with AD pathology and/or cognitive impairment. The groups were involved in MAP kinase signaling, metabolism, synaptic function, and the extracellular matrix (Feb 2022 news). While that cross-sectional study pointed to biological processes related to AD pathogenesis, it had but a single analysis timepoint, and therefore was unable to unearth changes afoot long before symptoms arise.
To fill this gap, the researchers turned to the dominantly inherited AD network (DIAN). It includes carriers of autosomal-dominant mutations in APP, PS1, or PS2, who are destined to develop AD, and their noncarrying siblings. Using targeted, quantitative mass spectrometry, the scientists measured a curated set of 59 proteins in the CSF of 286 mutation carriers and 184 noncarriers. They spanned six decades of disease progression from 39 years prior to 24 years after their expected age of symptom onset based on their family's mutation. Johnson told Alzforum that he curated the list of proteins based on findings from previous proteomics studies on sporadic AD, with an eye toward including diverse biological processes.
Of the 59 proteins, 33 significantly differed between the ADAD mutation carriers and noncarriers at some point along the disease continuum. They fit into five categories. The first comprised proteins of the so-called “matrisome,” APP and ApoE among them. Matrisome proteins mingle with the extracellular matrix, and some have been spotted within Aβ plaques (Aug 2019 news). The researchers had previously tied a collection of matrisome proteins to sporadic AD in an analysis of postmortem brain samples.
In ADAD, the matrisome members SMOC1 and SPON1 were elevated in CSF some 30 years prior to symptom onset, preceding changes in Aβ42/Aβ40 and p-tau217 in the CSF (image below). While SMOC1 remained high throughout the course of disease, SPON1 sunk back down to levels of noncarriers after about seven years.

Early Riser. Thirty years before symptoms start, SMOC1 begins its rise (bottom) in mutation carriers (red) but not noncarriers (blue). For comparison, see Aβ42/Aβ40 ratio (top). [Courtesy of Johnson et al., Nature Medicine, 2023].
Following the matrisome, a second category of proteins started to move. This wave was marked by 14-3-3 proteins, which function in synapses; as well as proteins needed for glycolytic metabolism. The 14-3-3 proteins crept upward between 26 to 22 years before onset and skyrocketed around eight years prior. The metabolism proteins waxed and waned throughout disease, peaking around 17 years prior to onset, then returning to levels of noncarriers before spiking again as carriers began to show symptoms.
The researchers proposed that the initial metabolic boost may have reflected a beneficial, compensatory response to an originating insult, as neuroprotective proteins rose at the same time as well. Interestingly, this coincided with a period of improved cognition in carriers relative to noncarriers, suggesting that for a time, the protection worked.
Russell Swerdlow of Kansas University Medical Center in Kansas City agreed that the first spike in glucose metabolism likely reflects some form of compensation, perhaps an attempt to maintain bioenergetic homeostasis, or, more likely to his mind, to synthesize macromolecules. “Why this peak in glucose metabolism-related proteins goes down after it initially rises, then rises again down the line, is probably an important clue to the disease, even if it is not driving familial AD,” he wrote.
Manu Goyal of Washington University in St. Louis raised yet another possible explanation for the early metabolic spike. He noted that it coincides with both the start of amyloid plaque development as well as improved cognition. “To me this parallels studies in mouse models where soluble amyloid is associated with neuronal hyperexcitability,” Goyal wrote (comment below).
Between 20 to 10 years prior to onset, a third wave of proteomic changes swelled in the CSF. At around 19 years prior to disease onset, total tau and p-tau205 started to rise, followed by an upturn in soluble TREM2. As mutation carriers entered their last symptom-free decade, neurofilament light (NfL) rose, indicating axonal damage. Six years prior to onset, inflammatory proteins, including osteopontin and YKL-40, ramped up, and soluble TREM2 increased more steeply than before. Following this fourth, pro-inflammatory surge, a fifth and final flurry of changes was marked by plummeting neurosecretory proteins, and a second, transient rush of sugar metabolism proteins. During this last phase, proteomic flux coincided with cognitive decline, dropping glucose metabolism as measured by FDG-PET scans, and brain atrophy (image below).

Alzheimer's Proteome Trajectory. Distinct waves of proteomic changes occurred over six decades of the AD pathogenesis cascade. [Courtesy of Johnson et al., Nature Medicine, 2023.]
Might some of these CSF proteins be biomarkers? Indeed, the researchers found that at both 20 and 10 years prior to onset, a composite of the 33 proteins rivaled classic AD biomarkers in deciphering carriers from noncarriers. Twenty years out, SMOC1 alone outperformed CSF p-tau and Aβ42/40, casting it as a sensitive detector of the first inklings of AD pathogenesis.
“This extremely exciting and important study provides new information about the time course of CSF proteomic changes associated with … the clinical progression of ADAD,” wrote Eric Reiman of Banner Alzheimer’s Institute in Phoenix. “It introduces new opportunities to understand the molecular processes involved in the development of AD, the discovery of novel drugs for the treatment, secondary prevention, and primary prevention of AD, and the identification of persons who may benefit from relevant treatments at different preclinical and clinical stages of the disease.”
Indeed, Johnson plans to probe proteins flagged in the study, particularly SMOC1 and other matrisomal proteins, as markers or drug targets. He thinks these and other matrisomal proteins are involved in Ab plaque formation. Joachim Herz of University of Southwestern Medical Center in Dallas said he sees diagnostic value in monitoring proteomic changes in asymptomatic disease, but no new insight into disease mechanisms. “I think the key to understanding AD onset lies inside the cell, where it involves endolysosomal acidification and vesicular trafficking. If that goes awry, these secondary and post-secondary changes ensue,” he wrote. “I don’t see these proteomic changes as a direct result of this, rather as the consequence of a shift in metabolic capacity, waste disposal, and energy homeostasis of the neuronal-glial system.”
The hand-picked panel of 59 proteins analyzed here excludes proteins that would specifically detect endolysosomal dysfunction. Future studies will deploy large-scale proteomics to these samples, Johnson said.
Peripheral Proteomics
Walker’s plasma proteomics study was larger. It addressed a different question: How might the mid-life plasma proteome relate to risk of future dementia? To answer it, Walker, Coresh, and colleagues used large-scale proteomics to measure the abundance of 4,877 proteins in banked plasma samples from 10,981 participants in the Atherosclerosis Risk in Communities (ARIC) cohort. They then hunted for associations between each protein with dementia over a 25-year follow-up period, when nearly 2,000 participants were diagnosed with the disease. At plasma collection, participants averaged 60 years of age; 5,285 were in their 40s or 50s.
At baseline, 452 proteins linked to future dementia, but after adjusting for a slew of factors including demographics, cardiovascular risk, and ApoE4 status, only 26 remained significant. Growth/differentiation factor-15 (GDF-15) was most strongly tied to future dementia. The other 25 are involved in neuronal and/or synaptic function, innate and adaptive immune signaling, ubiquitination and autophagy, and the organization or degradation of the extracellular matrix.
To differentiate between proteins associated with early versus later dementia processes, the researchers looked for proteins tied to dementia within 15 years, versus proteins linked to dementia that cropped up 15 to 25 years after plasma samples were taken. In this segregated analysis, they identified six new plasma proteins—SERPINA3, IGFBP2, eGFR, FAP, GHR, and CBLN4—that were only tied to the more proximal dementia, growing their list of total dementia-related proteins to 32. These new proteins rose at a time when pathogenesis was likely well underway in the brain. They were involved in coagulation and inflammation. In contrast, matrix metalloproteases, the unfolded protein response, bile acid biosynthesis, and granulocyte adhesion were among the pathways cranked up in people who were 15-25 years out from diagnosis. GDF15 was the only protein significantly elevated across both timeframes.
Using protein co-expression analysis, the researchers identified distinct immune and vascular pathways at work during the two different time frames prior to dementia diagnosis.
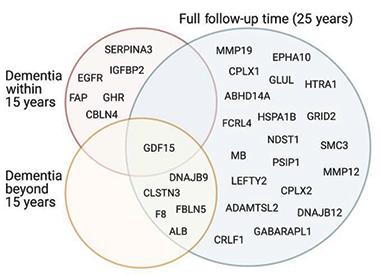
Tied to Dementia. Plasma proteins associated with dementia at any time during the 25-year follow-up (blue), within 15 years (pink) of dementia onset, or prior to 15 years before symptom onset (yellow) partially overlapped. [Courtesy of Walker et al., Science Translational Medicine, 2023.]
“Together, these results suggest a multidecade immunologic signature characterized by early involvement of JAK-STAT and Toll-like receptor signaling, leukocyte activation, and extracellular matrix degradation, followed by more prominent alteration of complement and coagulation protein networks later in the disease course,” the authors wrote.
The researchers investigated other cohorts in numerous ways to learn how plasma proteins influence dementia risk. For example, among participants in the European Medical Information Framework for AD (EMIF-AD) study, 12 of the 26 dementia-related proteins were differentially expressed in people with AD versus controls, or were associated with progression from MCI to dementia. Seven of the plasma hits correlated with amyloid status, while others only tracked with CSF markers of neuroinflammation. For example, the top hit, GDF-15, only tracked with CSF YKL-40.
Walker’s group is currently exploring these top hits. GDF-15, aka macrophage inhibitory cytokine-1, was hardly detectable in postmortem brain samples or in CSF, suggesting it either promotes, or at least strongly indicates, dementia-related processes from outside of the brain, Walker told Alzforum. Reportedly induced by stress, infection, and inflammation, GDF-15 is highly expressed in senescent cells and is thought to suppress immune responses (reviewed in Pence et al., 2022). The cytokine also bungles cancer immunotherapies by thwarting T cell recruitment to tumors (Haake et al., 2023). Walker wondered whether it might be upregulated as a compensatory response to ongoing peripheral inflammation, or perhaps, might be squelching T cell infiltration into the brain.

Mid-life Crisis. Of 32 dementia-related proteins, these 15 are undergoing further study. The image includes descriptions of their primary molecular functions. [Courtesy of Walker et al., Science Translational Medicine, 2023.]
Walker is intrigued by the proteostasis, stress response, and autophagy proteins tied to dementia. Unlike GDF-15, some of these proteins correlated with CSF biomarkers of AD. One, HSPA1B, was up in AD brain samples. This suggests that their rise in plasma could reflect ongoing dementia-related processes in the CNS as well as the periphery, Walker said.
Finally, echoing findings from the ADAD study, proteins involved in the making and breaking of the extracellular matrix cropped up in the plasma. However, Walker interprets their rise there as an indicator of cardiovascular problems, which themselves can boost dementia risk.
This interpretation meshes with the results of a risk-prediction analysis. The scientists found that, relative to a mix of demographics, ApoE4, and cardiovascular factors, a composite of their dementia-related plasma proteins barely improved prediction of future dementia—from 77 to 78 percent. This might be because some of the proteins influence dementia via cardiovascular risk. Furthermore, given the heterogeneity of both the participants and the way dementia was ascertained in the large community cohort, the low predictive value is not surprising, Walker added.
Despite differences in design and scope between the two proteomics studies, Johnson noted commonalities. Both pinpointed a relationship between extracellular matrix and future neurodegenerative disease. “These common findings highlight, perhaps, the most salient take-home message from both studies, which is that AD is a chronic disease with a long pre-symptomatic course that is likely also systemic in late-onset cases,” he commented to Alzforum. “Therefore, therapeutic interventions for AD and ADRD through medication and/or lifestyle changes will likely be most impactful when implemented in a preventive paradigm, similar to most other chronic diseases in medicine.”
Walker agreed. He hopes the plasma protein findings will identify therapeutic targets that can stem neurodegeneration from the periphery. For example, if the goal is to enhance TREM2 signaling within the brain, perhaps this could be achieved by modulating peripheral inflammation, he suggested.—Jessica Shugart
References
News Citations
- Proteomics Highlight Alzheimer’s Changes in Matrisome, MAPK Signaling
- ApoE4 Glia Bungle Lipid Processing, Mess with the Matrisome
Paper Citations
- Pence BD. Growth Differentiation Factor-15 in Immunity and Aging. Front Aging. 2022;3:837575. Epub 2022 Feb 9 PubMed.
- Haake M, Haack B, Schäfer T, Harter PN, Mattavelli G, Eiring P, Vashist N, Wedekink F, Genssler S, Fischer B, Dahlhoff J, Mokhtari F, Kuzkina A, Welters MJ, Benz TM, Sorger L, Thiemann V, Almanzar G, Selle M, Thein K, Späth J, Gonzalez MC, Reitinger C, Ipsen-Escobedo A, Wistuba-Hamprecht K, Eichler K, Filipski K, Zeiner PS, Beschorner R, Goedemans R, Gogolla FH, Hackl H, Rooswinkel RW, Thiem A, Roche PR, Joshi H, Pühringer D, Wöckel A, Diessner JE, Rüdiger M, Leo E, Cheng PF, Levesque MP, Goebeler M, Sauer M, Nimmerjahn F, Schuberth-Wagner C, von Felten S, Mittelbronn M, Mehling M, Beilhack A, van der Burg SH, Riedel A, Weide B, Dummer R, Wischhusen J. Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment. Nat Commun. 2023 Jul 20;14(1):4253. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Johnson EC, Bian S, Haque RU, Carter EK, Watson CM, Gordon BA, Ping L, Duong DM, Epstein MP, McDade E, Barthélemy NR, Karch CM, Xiong C, Cruchaga C, Perrin RJ, Wingo AP, Wingo TS, Chhatwal JP, Day GS, Noble JM, Berman SB, Martins R, Graff-Radford NR, Schofield PR, Ikeuchi T, Mori H, Levin J, Farlow M, Lah JJ, Haass C, Jucker M, Morris JC, Benzinger TL, Roberts BR, Bateman RJ, Fagan AM, Seyfried NT, Levey AI, Dominantly Inherited Alzheimer Network. Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer's disease. Nat Med. 2023 Aug;29(8):1979-1988. Epub 2023 Aug 7 PubMed.
- Walker KA, Chen J, Shi L, Yang Y, Fornage M, Zhou L, Schlosser P, Surapaneni A, Grams ME, Duggan MR, Peng Z, Gomez GT, Tin A, Hoogeveen RC, Sullivan KJ, Ganz P, Lindbohm JV, Kivimaki M, Nevado-Holgado AJ, Buckley N, Gottesman RF, Mosley TH, Boerwinkle E, Ballantyne CM, Coresh J. Proteomics analysis of plasma from middle-aged adults identifies protein markers of dementia risk in later life. Sci Transl Med. 2023 Jul 19;15(705):eadf5681. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Amsterdam UMC, loc. VUmc
Maastricht University; VU University Medical Centre
Johnson et al. show beautiful analyses of CSF proteomic alterations in relation to estimated year of disease onset (EYO), providing, for the first time, in-depth insight into when, and which, molecular processes are dysregulated in autosomal-dominant AD.
The panel measured with selected reaction monitoring mass spectrometry included 59 proteins, which the authors, in their previous studies, had identified to be altered in brain tissue and to be related to pathology in sporadic AD. Previous CSF studies that investigated associations with EYO were restricted to just a few proteins that were measured with ELISAs.
A particular strength of this study is that the proteins selected seem to have distinct relationships with EYO, which are specific to CSF amyloid, tau, and imaging and clinical outcomes. These results suggest that different processes become abnormal in five different stages of the disease: in the very early stage SPON1 and SMOC1 were increased together with decrease in the Aβ42/40 ratio. These proteins have been reported to be increased in sporadic AD in a number of different studies (e.g., by Sung et al., 2023; Tijms et al., 2023; Visser et al., 2022). …More
The second group included proteins with higher levels than in noncarriers, which were related to proteins in glycolytic metabolism. This was followed by a third stage, in which tau increased together with cognitive impairment. The fourth stage included proteins related to immune activation, while stage 5 was characterized by decreased levels of other glycolytic proteins and synaptic proteins.
This paper is a milestone, providing a new view as to how AD develops. This will definitely be further refined when more proteins are measured.
Also, because these proteins were selected based on sporadic AD, their relationship with ADAD further validates their role in AD pathogenesis. A major question remains as to how early these proteins change in sporadic AD, where it is not possible yet to define an EYO.
Plasma proteomics may provide further insight, as this fluid is more easily obtained.
Indeed, Walker et al. took this approach to look into an enormous longitudinally followed cohort of more than 10,000 individuals whose cognition was normal at the start of the study. These individuals were followed for more than 25 years. Interestingly, the authors found that protein levels associated with MAPK signaling were increased 25 years before dementia onset. In the Johnson study, these proteins were increased in early AD as well. Although the Walker et al. study did not mention SMOC1, they did find that alterations in proteins related to the extracellular matrix were predictive for future dementia. It would be of major interest to learn how the plasma proteins associate with plasma p-tau and amyloid markers in the same cohort.
References:
Sung YJ, Yang C, Norton J, Johnson M, Fagan A, Bateman RJ, Perrin RJ, Morris JC, Farlow MR, Chhatwal JP, Schofield PR, Chui H, Wang F, Novotny B, Eteleeb A, Karch C, Schindler SE, Rhinn H, Johnson EC, Oh HS, Rutledge JE, Dammer EB, Seyfried NT, Wyss-Coray T, Harari O, Cruchaga C. Proteomics of brain, CSF, and plasma identifies molecular signatures for distinguishing sporadic and genetic Alzheimer's disease. Sci Transl Med. 2023 Jul 5;15(703):eabq5923. PubMed.
Tijms BM, Vromen EM, Mjaavatten O, Holstege H, Reus LM, vanderLee SJ, Wesenhagen K, Lorenzini L, Vermunt L, Venkatraghavan V, Tesi N, Tomassen J, denBraber A, Goossens J, Vanmechelen E, Barkhof F, Pijnenburg YA, vanderFlier WM, Teunissen CE, Berven F, Visser PJ. Large-scale cerebrospinal fluid proteomic analysis in Alzheimer's disease patients reveals five molecular subtypes with distinct genetic risk profiles. 2023 May 11 10.1101/2023.05.10.23289793 (version 1) medRxiv.
Visser PJ, Reus LM, Gobom J, Jansen I, Dicks E, van der Lee SJ, Tsolaki M, Verhey FR, Popp J, Martinez-Lage P, Vandenberghe R, Lleó A, Molinuevo JL, Engelborghs S, Freund-Levi Y, Froelich L, Sleegers K, Dobricic V, Lovestone S, Streffer J, Vos SJ, Bos I, ADNI, Smit AB, Blennow K, Scheltens P, Teunissen CE, Bertram L, Zetterberg H, Tijms BM. Cerebrospinal fluid tau levels are associated with abnormal neuronal plasticity markers in Alzheimer's disease. Mol Neurodegener. 2022 Mar 28;17(1):27. PubMed. Correction.
University of Toronto
In the age of big data, it has become an almost impossible task to genuinely assess papers reporting on thousands of samples, short of repeating the analyses. Although with resource manuscripts like this, the long-term impact can be difficult to gauge initially, the work by Walker et al. is groundbreaking beyond its scale.
In addition to providing testable hypotheses, by putting a spotlight on specific blood proteins, including GDF15 and SERPINA3, the study adds to the concept that the earliest changes in the levels of blood proteins, which may be helpful for dementia risk prediction, can be obscured by the time the disease progresses to dementia.
Washington University School of Medicine
This new DIAN study is indeed fascinating. There has been prior research suggesting that glycolytic metabolism is specifically altered early in Alzheimer's disease. What makes studies like these in autosomal-dominant Alzheimer's disease highly impactful is their ability to tease apart the timing of different events, including the timing related to metabolism.
It is striking to see that enzymes associated with glycolysis transiently rise in the CSF almost two decades prior to expected symptom onset and again near symptom onset. I agree with the authors that other studies will be needed to understand why this is occurring and in what cells. It is also difficult to know how CSF proteins reflect metabolic flux in the brain. However, these data suggest some intriguing possibilities. …More
The first rise in glycolytic proteins appears to occur just as amyloid plaques start developing and coincides with a transient period of improved cognition. There are several ways to potentially explain this, but to me this parallels studies in mouse models where soluble amyloid is associated with neuronal hyperexcitability. In contrast, the second rise in glycolytic enzymes occurs closer to symptom onset, which might reflect various processes including a glial response as suggested by the authors.
Regardless, I think this study strongly supports the need to investigate brain metabolism not only as a result of neurodegeneration, but potentially as a key element in the pathogenesis of Alzheimer's disease.
Boston University School of Medicine
This ADAD CSF proteomic study is an asset to the AD community as it’s based on a collection of samples over the course of six decades. The authors showed changes in proteins from CSF associated with biomarker and pathological changes of AD. Especially protein changes in the prodromal stage of the disease and patterns of target pathways are of interest.
The first target pathway highly associated with Aβ plaque in AD 30 years before disease onset is the “matrisome,” which has been previously identified as one of the major pathways in AD and APOE4 AD human brain in transcriptome (TCW et al., 2022) and proteome (Johnson et al., 2022). Within this category, the study highlighted two novel proteins, SMOC1 and SPON1, potentially involved in Aβ plaque formation. While SPON1 is temporarily activated for five to seven years, SMOC1 stays activated before and after disease onset, which could be promising as the earliest-detectable biomarker for AD.…More
After matrisome activation, it is also interesting to see that glycolytic metabolism and stress response pathways display pulsing patterns. Gycolytic metabolism especially pulses twice; once after matrisome and another right on the clinical onset when the immune activation pathway is elevated, indicating glial activation. Future studies on the potential connection between metabolic changes and neuronal stress response and glial activation and their mechanistic study can provide a better resolution of these temporal responses.
This study is a great reference for LOAD associated with amyloid and tau changes. For LOAD cases, when picturing our future, we can identify biomarkers isolating the risk group who requires treatments multiple decades earlier to prevent the disease, expecting a drug that we can easily take to prevent amyloid formation.
References:
Tcw J, Qian L, Pipalia NH, Chao MJ, Liang SA, Shi Y, Jain BR, Bertelsen SE, Kapoor M, Marcora E, Sikora E, Andrews EJ, Martini AC, Karch CM, Head E, Holtzman DM, Zhang B, Wang M, Maxfield FR, Poon WW, Goate AM. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022 Jun 23;185(13):2213-2233.e25. PubMed. BioRxiv.
Johnson EC, Carter EK, Dammer EB, Duong DM, Gerasimov ES, Liu Y, Liu J, Betarbet R, Ping L, Yin L, Serrano GE, Beach TG, Peng J, De Jager PL, Haroutunian V, Zhang B, Gaiteri C, Bennett DA, Gearing M, Wingo TS, Wingo AP, Lah JJ, Levey AI, Seyfried NT. Large-scale deep multi-layer analysis of Alzheimer's disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat Neurosci. 2022 Feb;25(2):213-225. Epub 2022 Feb 3 PubMed.
University of Kansas
The proteomics analysis performed in this paper, on CSF collected as part of the DIAN study, is a tour de force. There are a number of intriguing findings. I was very interested by the finding that certain protein levels differed between the mutation carriers and non-mutation carriers before any changes in Aβ or tau biomarkers were detected. As most APP mutations are in the APP gene, not an “Aβ” gene, and presenilin proteins act on APP protein, one should wonder whether in familial AD (FAD) currently unclear changes in APP-relevant biology lie upstream of Aβ.
There are two fascinating rises and falls in proteins relevant to glucose that play out over decades. The first one occurs long before symptoms develop. I would presume this represents an attempt to compensate, and to maintain homeostasis. Compensation for what, and homeostasis for what, is a mystery. Perhaps it reflects an attempt to maintain bioenergetic homeostasis. Maybe it reflects an attempt at macromolecule synthesis. My guess is it reflects the latter.…More
Why this peak in glucose metabolism-related proteins goes down after it initially rises, then rises again down the line, is probably an important clue to the disease, even if it is not driving the disease in FAD.
There are other compelling questions. I’d love to know how these glucose metabolism-relevant proteins are accessing the CSF. Are they secreted? Do they just spill into the extracellular space as cells die?
There is also the question of spatial contribution. What cells are producing these proteins, or supplying them to the CSF, and what brain regions are contributing? Finally, why would mutations in presenilin genes and the APP gene cause changes to the glucose metabolism module? I’d also love to know how the ways in which this pattern of change recapitulates, or fails to recapitulate, sporadic AD.
Arizona Alzheimer's Consortium
These extremely exciting and important studies provide new information about the time course of proteomic changes associated with predisposition to, and clinical progression of, autosomal dominant Alzheimer’s disease (ADAD) and sporadic AD. The papers illustrate the emerging value of proteomics in the study of AD and related dementias.
Johnson and colleagues capitalize on CSF samples and data from the Dominantly Inherited Alzheimer’s Network (DIAN), which continues to make pioneering contributions to unusually early detection, tracking, study, treatment, and prevention of AD, starting in cognitively unimpaired persons at virtually certain risk for the clinical onset of ADAD. The study leverages analyses by leaders in the study of AD proteomics from Emory University,…More
It provides new information about the sequence of proteomic changes associated with AD, starting more than 30 years before the estimated onset of symptoms, and their temporal relationships to the predisposition to amyloid plaques, to the ensuing pathophysiological changes of AD, and to the estimated ages at clinical onset. It introduces new opportunities to inform the molecular processes involved in the development of AD, the discovery of novel drugs for the treatment, secondary prevention, and primary prevention of AD, and the identification of persons who may benefit from relevant treatments at different preclinical and clinical stages of the disease.
It will be interesting to see the extent to which findings can be generalized to late-onset AD using legacy CSF samples, extended to the assessment of longitudinal trajectories, and expanded to proteomic findings in blood as we have begun to see in recent publications. Indeed, in their study, Walker and colleagues capitalized on extremely large longitudinal discovery and validation cohorts to establish the prognostic value of proteomic measures long before the onset of dementia. It will be interesting to see how blood-based biomarker measurements of AD and neurodegeneration in the cohorts can further clarify the extent to which these or other proteomic profiles could predict AD or non-AD dementias in the future.
We may have just started to see the emerging value of proteomics in the study of AD. Congratulations to everyone involved in this groundbreaking work.
Hong Kong University of Science & Technology
A key advantage of large-scale proteomic analysis is the unbiased examination of protein changes in diverse biological processes beyond well-studied biological pathways and pathological changes in disease conditions. Emerging evidence demonstrates that complex diseases, such as Alzheimer’s disease and dementia, involve the dysregulation of multiple body systems and biological processes, and are not restricted to the most affected organ (i.e., the brain in these diseases). Therefore, large-scale proteomic analysis is a powerful tool to screen for novel disease biomarkers and can provide a clearer and more holistic picture of how such diseases impact the human body.…More
In this study, the authors examined the associations of 4,877 plasma proteins with 25-year dementia risk in >10,000 middle-aged adults. By performing this large-scale proteomic analysis, they identified 32 plasma proteins that are associated with dementia and involved in different biological processes, such as proteostasis, immunity, synaptic functioning, and extracellular matrix organization. Notably, some of these dementia-associated plasma proteins—particularly those enriched in pathways related to peripheral immune response and proteostasis/autophagy—start to be dysregulated in midlife, 10–20 years before dementia onset. Therefore, these proteins can be used to predict dementia risk with up to 78 percent accuracy. These findings collectively suggest that peripheral biological processes are dysregulated in the early stages of dementia and AD, and therefore encompass important biomarkers for characterizing and predicting disease outcomes.
Such early stage dysregulation of peripheral biological processes in dementia and AD is not surprising. Our previous large-scale proteomic profiling of AD plasma also revealed hundreds of AD-associated blood proteins that are involved in peripheral immune response, apoptosis, inflammation, etc.; some of these blood proteins start to be dysregulated upon disease progression when individuals still have normal cognitive functioning (Jiang et al., 2022). Moreover, certain blood proteins, such as IGFBP-2 and CLU, can be used to predict the risk of AD and the rate of cognitive decline (Sattlecker et al., 2014). Therefore, these findings together with the present study suggest that the dysregulation of peripheral biological processes, which may or may not be associated with the typical pathological changes of AD (i.e., ATN-related brain pathologies), is a key characteristic of early stage AD. Hence, identifying such blood biomarkers could advance our understanding of the progression of AD as well as facilitate the early detection and staging of the disease.
Another interesting aspect of the present study is that the authors integrated proteomic and genetic data to perform Mendelian randomization analysis of the plasma proteome. This identified SERPINA3 as a potential disease-causing factor of AD. Notably, a recent Mendelian randomization study also revealed that peripheral CD33 is causally associated with AD risk (Gu et al., 2022). Thus, the present study potentially demonstrates an alternative pathophysiological mechanism of AD; namely, that peripheral dysfunctions of cells and pathways are potential triggers of AD. Thus, future investigation into the underlying pathological mechanisms could provide insights for the development of novel therapeutic strategies for the disease.
In summary, this study is a good example of the utility of large-scale proteomic analysis for identifying biomarkers and therapeutic targets for AD. Proteomic profiling in additional cohorts from different centers and/or ethnic populations will corroborate the capability of these biomarkers to reflect or contribute to the progression of AD, which will eventually benefit diagnostic and therapeutic development for the disease.
References:
Jiang Y, Zhou X, Ip FC, Chan P, Chen Y, Lai NC, Cheung K, Lo RM, Tong EP, Wong BW, Chan AL, Mok VC, Kwok TC, Mok KY, Hardy J, Zetterberg H, Fu AK, Ip NY. Large-scale plasma proteomic profiling identifies a high-performance biomarker panel for Alzheimer's disease screening and staging. Alzheimers Dement. 2021 May 25; PubMed.
Sattlecker M, Kiddle SJ, Newhouse S, Proitsi P, Nelson S, Williams S, Johnston C, Killick R, Simmons A, Westman E, Hodges A, Soininen H, Kłoszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, the AddNeuroMed Consortium, Dobson RJ. Alzheimer's disease biomarker discovery using SOMAscan multiplexed protein technology. Alzheimers Dement. 2014 Apr 24; PubMed.
Gu X, Dou M, Cao B, Jiang Z, Chen Y. Peripheral level of CD33 and Alzheimer's disease: a bidirectional two-sample Mendelian randomization study. Transl Psychiatry. 2022 Oct 3;12(1):427. PubMed.
View all comments by Nancy IpNational Institute on Aging
Johnson and colleagues have performed an impressive study that significantly advances our understanding of the time course of specific proteomic changes in Alzheimer’s disease. Better understanding the earliest disease-specific biological perturbations is key to developing diagnostics and therapeutics that target the insidious prodrome of AD. In this study, the authors evaluate a subset of 59 proteins previously established as disease-associated in brain tissue studies in CSF, specifically focusing on patients with autosomal-dominant genetic risk for disease associated with increased production of the Aβ42 and early brain Aβ plaque deposition.…More
Their primary finding, that SMOC1 was elevated in the CSF decades prior to disease onset, is particularly striking, as our recent publication identified SMOC1 among four proteins (additionally including NOG, APCS, and NTN1) highly discriminative of AD and control brain tissue in sporadic AD (Figure 1 in Roberts et al., 2023).
Figure 1. Levels of SMOC1 in middle frontal gyrus brain tissue. Comparison of middle frontal gyrus levels of SMOC1, quantified by SomaLogic aptamer-based proteomics, between Alzheimer’s disease (red) and control (blue) participants in the Baltimore Longitudinal Study of Aging (BLSA) and Religious Orders Study (ROS). Protein levels are on the y-axis in relative fluorescence units (RFUs). Statistical significance was calculated using sex- and age-adjusted proportional odds models. Horizontal lines within each box represent the mean, and error bars represent the standard deviation. ***p < 0.001.
This finding corroborates multiple studies that have found SMOC1 to be an amyloid-associated protein, reliably found among the most highly-enriched proteins in amyloid plaques at autopsy (Bai et al., 2020). Building on work identifying SMOC1 in CSF as discriminating asymptomatic individuals with Alzheimer’s pathology from those without (Watson et al., 2023), this work sets the stage for further evaluation of SMOC1 in other preclinical populations, including APOE ε4 allele carriers and other tissue matrices, to further our understanding of its pathogenic role and potential utility as a noninvasive preclinical AD biomarker.
This approach, in terms of studying earlier preclinical populations to not only identify predictive biomarkers but also the earliest perturbations related to disease pathogenesis, has been of interest for our group following our study of brain tissue proteomic alterations in young (mean age 39 years) APOE ε4 carriers that were recapitulated in two cohorts of late-onset AD (Roberts et al., 2023). Similar to some of the alterations observed in Johnson et al.’s study, we observed early increases of multiple proteins associated with tyrosine kinase signaling, the inflammatory response, and glycolytic pathways in young APOE ε4 carriers, and decreases in AD tissue. In populations at genetic risk for AD, it remains to be elucidated whether such early alterations reflect drivers of disease processes or a compensatory response to initial pathology. Johnson et al. provide evidence that at least a subset of their identified proteins may be compensatory and neuroprotective, given that their elevation conferred a period of improved cognitive performance in mutation carriers only, further underscoring the importance of identifying proteomic changes at multiple time points in association with neurocognitive testing and neuroimaging.
Overall, this paper marks a substantial advance in our understanding of early pathology-associated changes in AD. Further functional characterization of the protein changes observed, as well as additional work in blood samples and other preclinical populations more closely paralleling the LOAD population, will move the field closer to identifying novel preclinical therapeutics.
—NIH Reseearch Fellow Jackson Roberts is the co-author of this comment.
References:
Roberts JA, Varma VR, Candia J, Tanaka T, Ferrucci L, Bennett DA, Thambisetty M. Unbiased proteomics and multivariable regularized regression techniques identify SMOC1, NOG, APCS, and NTN1 in an Alzheimer's disease brain proteomic signature. NPJ Aging. 2023 Jul 6;9(1):18. PubMed.
Bai B, Wang X, Li Y, Chen PC, Yu K, Dey KK, Yarbro JM, Han X, Lutz BM, Rao S, Jiao Y, Sifford JM, Han J, Wang M, Tan H, Shaw TI, Cho JH, Zhou S, Wang H, Niu M, Mancieri A, Messler KA, Sun X, Wu Z, Pagala V, High AA, Bi W, Zhang H, Chi H, Haroutunian V, Zhang B, Beach TG, Yu G, Peng J. Deep Multilayer Brain Proteomics Identifies Molecular Networks in Alzheimer's Disease Progression. Neuron. 2020 Mar 18;105(6):975-991.e7. Epub 2020 Jan 8 PubMed.
Watson CM, Dammer EB, Ping L, Duong DM, Modeste E, Carter EK, Johnson EC, Levey AI, Lah JJ, Roberts BR, Seyfried NT. Quantitative Mass Spectrometry Analysis of Cerebrospinal Fluid Protein Biomarkers in Alzheimer's Disease. Sci Data. 2023 May 9;10(1):261. PubMed.
Roberts JA, Varma VR, Candia J, Tanaka T, Ferrucci L, Bennett DA, Thambisetty M. Unbiased proteomics and multivariable regularized regression techniques identify SMOC1, NOG, APCS, and NTN1 in an Alzheimer's disease brain proteomic signature. NPJ Aging. 2023 Jul 6;9(1):18. PubMed.
View all comments by Madhav ThambisettyMake a Comment
To make a comment you must login or register.