TAPAS Anyone? PyroGlu-Aβ Vaccine Shrinks Plaques in Mice
Quick Links
Looking to donanemab, researchers want more ways to target the pyroglutamate form of Aβ onto which this antibody latches. At the 16th International Conference on Alzheimer’s and Parkinson’s Diseases held in Barcelona, Spain, and online, Thomas Bayer, University Medical Center Göttingen, Germany, described how the new antibody TAP01 binds a β-hairpin structure in soluble pyroGlu-Aβ. It does not bind plaques. The scientists made a vaccine called N-Truncated Amyloid Peptide Antibodies (TAPAS). It mimics the antigen. Mouse amyloidosis models treated with the antibody or the vaccine accumulated fewer plaques, lost fewer neurons, and better preserved brain glucose metabolism and memory than did controls. Bayer believes either could be an attractive option to treat AD. None of the immunotherapies being tested in trials bind this epitope.
- Peptide dubbed TAPAS mimics a unique hairpin fold in pyroGlu-Aβ.
- Antibody TAP01 binds the hairpin in soluble oligomers but not plaques.
- Both vaccine and antibody reduced plaque load in mice.
- No current immunotherapy binds this epitope.
Also at AD/PD, Marija Vukicevic of AC Immune, Lausanne, Switzerland, described an improved version the amyloid vaccine ACI-24, which also targets pyroglu-Aβ. The company is about to begin clinical testing.
“These new findings are relevant and important for what I see as the future of anti-amyloid immunotherapy—namely, the development of safe and effective active vaccines to prevent or lower cerebral plaque deposition and keep it at bay long-term,” wrote Cynthia Lemere, Brigham and Women’s Hospital, Boston (comment below).
Pyroglutamate-modified Aβ is a truncated, stickier, and toxic version of Aβ. It is highly prone to oligomerize and seed plaques (reviewed by Bayer, 2021; Mar 2013 conference news; Apr 2008 conference news). Eli Lilly’s donanemab targets pyroGlu-Aβ within plaques, shrinking them and even slowing cognitive decline in the Phase 2 TRAILBLAZER-ALZ trial (Aug 2018 conference news; Jan 2021 news). The fly in the ointment? One in four treated people developed amyloid-related imaging abnormalities, aka ARIA, either as localized edema near blood vessels or mini hemorrhages.
To skirt these side effects, Bayer and colleagues looked for antibodies that target soluble amyloid rather than plaques. They found TAP01, which selectively bound oligomers of truncated forms, including pyroGlu-Aβ and Aβ4-42, but not aggregates (Antonios et al., 2015). At AD/PD, he reported that a humanized TAP01 also targets pyroGlu-Aβ oligomers but not plaques. Some of the findings were published last year (Bakrania et al., 2021).

Copy That. The cyclic Aβ1-14 peptide (blue), aka TAPAS, adopts an almost identical hairpin structure to the one (gray) bound by the TAP01 antibody. [Courtesy of Gareth Hall, University of Leicester.]
To scrutinize how TAP01 embraces pyroGlu-Aβ, Gareth Hall, University of Leicester, U.K., used x-ray crystallography. He found that the antibody hugged the 10 N-terminal amino acids of the truncated Aβ, which had folded into a unique β-hairpin. Inspired by this conformation, the researchers synthesized TAPAS, a cyclic Aβ1-14 peptide that locked into an almost identical β-hairpin (see image at right). Indeed, TAP01 bound the TAPAS hairpin.
Because this epitope had not been reported, Bayer wondered if the current batch of antibodies in clinical trials recognized it. None did; not solanezumab, lecanemab, bapineuzumab, not even those specific for pyroGlu-Aβ, including donanemab, ProBioDrug 6-1-6, and ProBioDrug 24-2-3 (Piechotta et al., 2017).
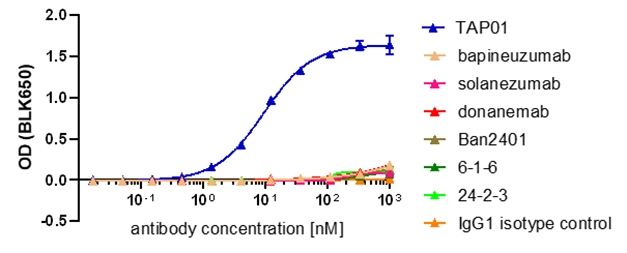
Unique Epitope. Only TAP01 (blue) bound to the TAPAS β-hairpin. [Bakrania et al., 2021, Molecular Psychiatry.]
Like TAP01, serum antibodies from mice injected with TAPAS bound the cyclic peptide in vitro, but not plaques in cortical slices from AD cases. “When pyroglutamate Aβ is soluble, we can detect the oligomers, but as soon as they aggregate into plaques, the hairpin epitope is either buried or no longer present for TAP01 or TAPAS antiserum to bind,” Bayer told Alzforum.
With the TAP01 antibody and TAPAS peptide in hand, the researchers asked how they affect plaque pathology in vivo. Yvonne Bouter of UMC Göttingen injected TAP01 into five 1.5-month-old 5xFAD mice weekly for 12 weeks, and the TAPAS vaccine into five 2-month-old mice that received biweekly boosters for two months. She scanned the mice at 4.5 months using amyloid and FDG microPET. Compared to controls, passively immunized mice had less cortical and hippocampal plaque load. Vaccinated mice also had fewer plaques and their glucose metabolism was higher (see image below). “Even though 5xFAD mice do not have much pyroglutamate Aβ, neutralizing this minute amount contributed to reduced plaque load,” Bayer said.
“It would be very interesting to know if his vaccine can clear existing plaques or if that’s even relevant,” noted Lemere. “It might be OK to just block the formation of N-terminally truncated Aβ oligomers, including pGlu3 and 4-X Aβ species,” she wrote.

Less Plaque, More Activity. PET scans for amyloid (top) and FDG (bottom) of untreated (left) and treated 5xFAD mice (right) reveal that TAPAS tempers amyloid plaques and preserves glucose metabolism in the cortex (C), hippocampus (Hc), thalamus (T), hypothalamus (H), and amygdala (A). TAP01 had similar but weaker effects (not shown). [Courtesy of Yvonne Bouter, University Medical Center Göttingen.]
What about neurodegeneration and memory? The researchers turned to Tg4-42 mice, which Bayer engineered to express Aβ4-42. They injected five 3-month-old mice with TAP01 weekly for 12 weeks and five 2-month-old mice with the TAPAS vaccine biweekly for two months, then monthly for two months. Tg4-42 mice develop noticeable neuron loss and behavioral deficits between three to six months as neurotoxic Aβ aggregates build up. Stereological analysis of hippocampal slices revealed that mice given either treatment retained more neurons than did controls. Both sets of mice found a hidden platform in a water maze faster than did controls.
Bayer said TAP01 is ready to move into the clinic, and is now in the hands of Preeti Bakrania at LifeArc, Center for Therapeutics Discovery, Stevenage, England, who was co-first author on the Molecular Psychiatry paper with Hall and Bouter. If passive immunotherapy proves to be safe in people, Bayer said they would focus on the vaccine because it would be cheaper and easier to administer. Meanwhile, he is optimizing TAPAS for use in people and hopes a trial will start within two years.
A Better ACI-24 Goes After PyroGlu-Aβ
Another pyroglu-Aβ vaccine, AC Immune’s ACI-24, uses an antigen from the N-terminal of Aβ. It has been tested in a Phase 2 trial in AD and a Phase 1 study in AD in Down’s syndrome. A more antigenic formulation, debuted at a Down’s syndrome forum last year (May 2021 news), uses non-Aβ peptides to rile up the immune system. “The peptide sequences originate from antigens to which humans are commonly exposed, such as tetanus, and provide T-cell help without engaging Aβ-specific T-cells to ensure a strong, boostable, and maintained anti-Aβ antibody response,” AC Immune’s Vukicevic wrote to Alzforum. The same strategy is being pursued for other Aβ vaccines (Aug 2021 conference news).
In Barcelona, Vukicevic elaborated on the new ACI-24's ability to generate antibodies against pyroGlu-Aβ and the full-length peptide, in mice and nonhuman primates. The findings were published last month (Vukicevic et al., 2022). In wild-type mice, the vaccine generated an anti-Aβ42 antibody titer 10-fold that of the version currently in trials. In mice and macaques, the antibodies recognized full-length Aβ, amyloid oligomers, and pyroGlu-Aβ. Monkeys that got the enhanced ACI-24 made 10 times the amount of anti-pyroGlu-Aβ antibodies as did primates who got the original Aβ peptide vaccine AN-1792, and 1,000-fold more than monkeys given the discontinued Aβ vaccine ACC-001. Antibodies generated by the older vaccines bound to N-terminal Aβ fragments, such as Aβ1-8, while ACI-24 antibodies preferred fragments lacking the first two to four amino acids, such as Aβ3-10 and Aβ4-11. This difference might explain why the ACI-24 antibodies bound so well to pyroGlu-Aβ.
Vukicevic said the company will test the updated ACI-24 in a Phase 1b/2 trial in prodromal AD, starting within the next few months, mostly to find a safe and immunogenic dose. The researchers will also measure effects on amyloid load via PET, and monitor phospho-tau181 and p-tau217 and clinical measures. Once the dose has been determined, a trial studying adults with Down’s is next.—Chelsea Weidman Burke
References
Therapeutics Citations
News Citations
- Can Dousing PyroGlu-Aβ Treat Alzheimer’s Disease?
- Keystone Drug News: Pyroglu Aβ—Snowball That Touches Off Avalanche?
- Four Immunotherapies Now Banish Amyloid From the Brain
- In Phase 2, Donanemab Curbs Cognitive Decline in Early Alzheimer’s
- In Down's Syndrome, Amyloid Vaccine Opens Door to Trials
- Up-and-Coming Immunotherapies Target Aβ and Tau
Research Models Citations
Paper Citations
- Bayer TA. Pyroglutamate Aβ cascade as drug target in Alzheimer's disease. Mol Psychiatry. 2022 Apr;27(4):1880-1885. Epub 2021 Dec 8 PubMed.
- Antonios G, Borgers H, Richard BC, Brauß A, Meißner J, Weggen S, Pena V, Pillot T, Davies SL, Bakrania P, Matthews D, Brownlees J, Bouter Y, Bayer TA. Alzheimer therapy with an antibody against N-terminal Abeta 4-X and pyroglutamate Abeta 3-X. Sci Rep. 2015 Dec 2;5:17338. PubMed.
- Bakrania P, Hall G, Bouter Y, Bouter C, Beindorff N, Cowan R, Davies S, Price J, Mpamhanga C, Love E, Matthews D, Carr MD, Bayer TA. Discovery of a novel pseudo β-hairpin structure of N-truncated amyloid-β for use as a vaccine against Alzheimer's disease. Mol Psychiatry. 2022 Feb;27(2):840-848. Epub 2021 Nov 15 PubMed.
- Piechotta A, Parthier C, Kleinschmidt M, Gnoth K, Pillot T, Lues I, Demuth HU, Schilling S, Rahfeld JU, Stubbs MT. Structural and functional analyses of pyroglutamate-amyloid-β-specific antibodies as a basis for Alzheimer immunotherapy. J Biol Chem. 2017 Jul 28;292(30):12713-12724. Epub 2017 Jun 16 PubMed.
- Vukicevic M, Fiorini E, Siegert S, Carpintero R, Rincon M, Lopez-Deber P, Piot N, Ayer M, Rentero I, Babolin C, Bravo-Veyrat S, Giriens V, Morici C, Beuzelin M, Gesbert A, Rivot S, Depretti S, Donati P, Streffer J, Pfeifer A, Kosco-Vilbois MH. An Amyloid beta (Abeta) vaccine that safely drives immunity to a key pathological species in Alzheimer’s disease: pyroglutamate Abeta. Brain Communications, Volume 4, Issue 1, 2022, fcac022 Brain Commun.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Ann Romney Center for Neurologic Diseases, Brigham and Women's Hospital and Harvard Medical School
I wish I had time to include in my plenary talk these two recent reports of active anti-amyloid vaccines that generate antibodies recognizing pGlu3 Aβ as well as other forms of Aβ. My talk was strictly focused on therapies specifically targeting pGlu3 Aβ. However, these new findings are relevant and important for what I see as the future of anti-amyloid immunotherapy—namely, the development of safe and effective active vaccines to prevent or lower cerebral plaque deposition and keep it at bay long-term. Because pGlu3 Aβ—an N-terminally-truncated and modified form of Aβ that aggregates quickly, resists degradation, and is neurotoxic—is the most common form of N-terminally modified Aβ species, it is gaining ground as a strong therapeutic target. In my talk, I provided some background regarding the formation of pGlu3 Aβ, and nonclinical data in mice and cell-culture studies that support the rationale for targeting pGlu3 Aβ as a treatment for Alzheimer’s disease. I focused on anti-pGlu3 Aβ antibody development (exploring different antibodies, IgG isotypes, and modifications to alter effector function) as well as the humanization of two antibodies for clinical development: donanemab (Eli Lilly’s anti-pGlu3 Aβ IgG1 mAb that showed promise in Phase 2 clinical trial and is now entering Phase 3 trials in MCI-AD and early AD and a secondary prevention trial) and PBD-C06 (Vivoryon Therapeutics’ anti-pGlu3 Aβ IgG1 mAb engineered to improve antibody stability, block C1q binding to reduce inflammation and possibly, ARIA, and reduce the anti-antibody response that was seen with donanemab). In addition, I discussed the development of an inhibitor of glutaminyl cyclase, the enzyme that causes the cyclization of Aβ 3-x to form pGlu3 Aβ. The drug, Varoglutamstat (or PQ912) was developed by Vivoryon Therapeutics and is now in Phase 2 clinical trials in Europe and the USA. [Of note: I receive anti-pGlu3 antibodies and other antibodies from Vivoryon for my NIH-funded work.]…More
Regarding AC immune’s AC-124 vaccine, the company originally incorporated Aβ 1-15 as the B cell epitope (based on previous work in the 2000s from Alon Monsonego’s lab and my lab) and a non-Aβ T cell epitope into their proprietary liposome-based vaccine platform. This vaccine has been shown to be safe and well-tolerated in Alzheimer’s patients and people with Down’s syndrome. In their “optimized ACI-24 vaccine,” AC Immune re-engineered the vaccine to expand the T helper epitopes to include those to which most of us have been previously exposed, such as tetanus, and therefore, should have a good T cell response. It worked. The titers were significantly elevated in C57BL/6 mice 22 and 36 days after the first dose compared to the original formulation of ACI-24. In both mice and nonhuman primates (NHPs), the optimized ACI-24 vaccine generated high titers to both Aβ 1-42 and pGlu3 Aβ, with higher titers to the former. Interestingly, the optimized ACI-24 vaccine generated higher titers to pGlu3 Aβ than AN1792 (the first active Aβ to go into clinical trials—using the full-length Aβ 1-42 peptide) in NHPs.
ACC-001, a second-generation active Aβ vaccine that used Aβ 1-7 as its B cell epitope, generated very low titers of antibodies recognizing pGlu3 Aβ in NHPs. The authors suggest that because their active vaccine is superior at generating anti-pGlu3 Aβ antibodies (as part of the diverse antibody response), it will provide better coverage in terms of preventing oligomerization (e.g., seeding) and deposition of Aβ into plaques. I agree but think we need to also consider the possibility that giving the vaccine to people with a lot of vascular amyloid may induce ARIA as the antibodies bind plaque and therefore will likely be effective at clearing amyloid in humans. It will be interesting to determine whether a slower induction of antibody responses via antigen presentation by T cells and B cell generation of antibodies will lower the risk of ARIA using active immunization. Ultimately, it is my hope that we will someday get to the point where we can prevent AD with an active vaccine. One caveat here, though, is that we are still not sure of the role of Aβ in the brain and therefore, removing non-modified Aβ (i.e., Aβ 1-42) may be risky. However, the ACI-24 vaccine is formulated to detect and bind β-pleated sheet structures so it should not bind well to monomeric Aβ and instead bind aggregates. Dr. Vukicevic presented data in support of this during her ADPD 2022 talk: NHP serum antibodies generated by the optimized ACI-24 bound to plaques in human AD brain sections. This seems like a good strategy for clearing plaques and preventing future oligomer and plaque formation.
Thomas Bayer’s group in Germany recently reported a novel and unique conformational epitope in the Aβ N-terminus that is able to generate antibodies against induce truncated Aβ peptides (called TAPAS). This lab has studied N-terminally truncated species of Aβ for many years, focusing primarily on pGlu3 Aβ and Aβ starting at residue 4. Previously, the lab generated an anti-amyloid antibody called TAP01 that bound to Aβ 4-x and pGlu3 Aβ small MW aggregates but not Aβ 1-42, and was effective at preventing disease in Tg4-42 and 5XFAD mice. Crystal structure analysis of the binding of the antibody to pGlu3 Aβ peptide revealed a novel pseudo beta-hairpin structure in the Aβ N-terminus that facilitated the binding to Aβ 4-x and pGlu3 Aβ. Bayer’s team then found a way to mimic this hairpin turn using a cyclized Aβ 1-14 peptide, which resulted in a similar three-dimensional conformational epitope. Next, they used the cyclized peptide to immunize mice, and found robust prevention of plaques and protection against changes in glucose metabolism in 5XFAD mice. In addition, the team showed that the active vaccine prevented neuron loss and cognitive decline when given prior to the onset of both in the Tg4-42 mouse model. Passive immunization studies with the humanized TAP01 mAb showed similar results. Intriguingly, the antibody does not bind plaque amyloid, which contains pGlu3 Aβ aggregates. Instead, it is more specific to small, soluble MW aggregates of N-terminally truncated Abeta species. Interestingly, current humanized anti-pGlu3 antibodies from Lilly (donanemab) and Vivoryon Therapeutics (PBD-C01) apparently do not bind this newly identified beta-hairpin 3D conformation which mimics monomeric pGlu3 Abeta, even though they do bind well to oligomeric and fibrillar pGlu3 Abeta. It is possible that the different antibodies recognize different conformational-specific epitopes within the N-termini of oligomers. It appears that a humanized version of Bayer’s antibody (TAP01_04) is in clinical development for the treatment of AD. It will be interesting to see if the antibody can reduce existing plaques or simply prevent the occurrence of new plaques and block the synaptotoxic effects of N-terminally truncated Abeta oligomers. It is quite possible that this antibody may avoid ARIA because it does not bind plaques. Time will tell!
About these papers, it is interesting that in one case—AC Immune’s vaccine—the vaccine generates a variety of antibodies that recognize full-length or pGlu3 Aβ quite well. And the antibodies bind plaques. That bodes well for plaque clearance but patients may incur ARIA when vascular amyloid is being cleared. On the other hand, the slow build-up of antibody titers and the engagement of the immune response by an active vaccine may help mitigate ARIA.
In Bayer’s vaccine/mAb, the target is N-terminally truncated Aβ with a specific three-dimensional conformation, and the antibodies bind small molecular-weight oligomers of these species but not plaques and not fibrillar forms of amyloid. Bayer shows prevention of plaque deposition, but I did not see any therapeutic studies, i.e., treating mice with lots of plaques at the start of treatment. It would be very interesting to know if his vaccine can clear existing plaques or if that’s even relevant. It might be OK to just block the formation of N-terminally truncated Aβ oligomers, including pGlu3 and 4-X Aβ species, and future plaques. As I said, by not binding plaques, it is quite possible that this strategy will result in less ARIA. In the end, perhaps we will need an anti-pGlu3 Abeta antibody or ACI-24 to clear existing plaques and an active vaccine like TAP01 to prevent the seeding of new oligomers and plaques going forward. Or, perhaps an active vaccine targeting pathogenic Abeta will be sufficient to prevent AD.
Make a Comment
To make a comment you must login or register.