Next Time You Spot That Familiar Face, Thank Your Temporal Pole
Quick Links
From across a crowded room, a familiar face leaps out instantaneously. This lightening-fast recognition may come courtesy of a small patch of neurons in the temporal pole—at least in monkeys. According to a paper in today’s Science, these neurons fire when a monkey views a face of another with which they’ve shared personal encounters, but not when viewing a “famous monkey” they have merely seen on screen. Led by Winrich Freiwald at The Rockefeller University, New York City, the study uncovers specialized circuitry that may explain the uncanny speed with which familiar faces are recognized.
- Neurons in the temporal pole fire fast when a monkey sees a picture of a familiar monkey’s face.
- These neurons stay mum in response to pictures of unfamiliar monkey faces—or human faces.
- The cells fire with surprising speed, suggesting a specialized pathway for familiar face recognition.
It also could shed light on prosopagnosia, a socially debilitating disorder in which a person cannot recognize faces of people they know. This symptom crops up in people with neurodegenerative diseases, including frontotemporal degeneration and Alzheimer’s. No longer being recognized by a parent with dementia is among the saddest experiences for caregivers.
The process of noticing a familiar face involves the rapid integration of sensory perception with stored memories associated with the face. A network of “face areas” is scattered throughout the superior temporal sulcus and inferotemporal (IT) cortex (Moeller et al., 2008; Freiwald and Tsao, 2010). Within the IT, the anteromedial (AM) face area is thought to be the crown jewel of this core face processing network.
This network has been well-described for encoding unfamiliar faces. However, Freiwald’s group previously singled out groups of neurons in two other regions that fired only in response to well-known faces. Using functional magnetic resonance imaging to observe which regions fired as a monkey observed images, the researchers reported that while regions such as the AM lit up in response to any face, neurons in the temporal pole and the perirhinal cortex fired only in response to familiar faces (Landi and Freiwald, 2017). Could these neurons handle familiar face recognition?
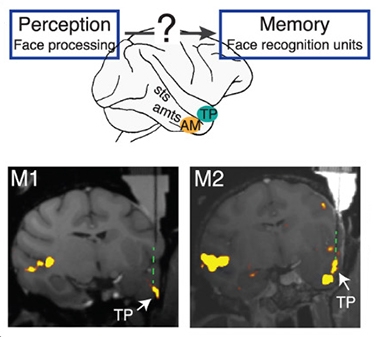
Flash of Familiarity. Face perception circuitry somehow connects with face memory. Neurons in the temporal pole (TP) and anteromedial face area (AM) activate in response to faces, but neurons in the TP react only to familiar faces. [Courtesy of Landi et al., Science, 2021.]
For the current study, first author Sofia Landi and colleagues took a closer look at how neurons in the temporal pole (TP) might facilitate rapid recognition of a familiar face. They used electrophysiological probes to monitor individual neuronal responses in the TP and the AM in two rhesus macaques, which they showed a series of 205 images of familiar or unfamiliar faces, bodies, and objects. They flashed 30 familiar and 30 unfamiliar human faces; 12 familiar and 72 unfamiliar monkey faces; as well as the monkey’s own face. The monkeys had met the familiar monkeys and humans in person many times, while they had seen images of the unfamiliar monkeys and people on screen before. In this way, the unfamiliar monkeys were more akin to faces of famous people commonly used in face-name recognition tasks. As the monkeys looked at the images, the researchers monitored the activity of 98 neurons in their TP and 130 neurons in their AM.
Neurons in the TP responded with remarkable specificity to familiar monkey faces. While some TP neurons lit up in only response to a single familiar face, others responded to multiple familiar faces. Conversely, most TP neurons remained silent when the monkey gazed upon a human face, regardless of personal familiarity. Familiar monkey faces evoked triple the response as unfamiliar faces in the TP population. In contrast, neurons in the AM fired in response to all faces, known or unknown, monkey or person.
Zeroing in on the particulars, the researchers found that the temporal pole neurons responded holistically to the face, rather than ramping up their responses linearly as more of the face was revealed. The inner face—eyes, nose, and mouth—was sufficient to evoke the response, and viewing the entire face or the rest of body did little to enhance it.
Finally, the researchers clocked the neurons’ responses. To their surprise, they found that temporal pole neurons spiked in response to familiar faces within 100 milliseconds, just as fast as AM neurons reacted to any face. This suggested that rather than lying downstream of the AM within the core facial recognition network, the TP neurons fired in response to familiar faces in parallel—and with astonishing speed. The findings cast these specialized cells as a new class of face memory neuron.
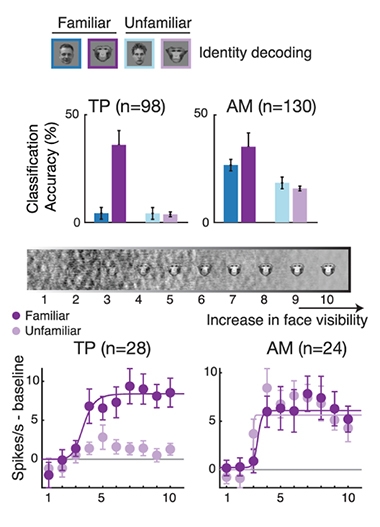
TP: Tuned to the Known. TP neurons reliably discriminate between familiar monkey faces (dark purple), while AM neurons discriminate between different faces in every category. As an image of a monkey face becomes sharper (middle bar), TP neurons fire off in response to familiar faces at a sharp threshold (below), while AM neurons do so regardless of familiarity. [Courtesy of Landi et al., Science, 2021.]
The authors propose two parallel pathways of face memory. The first one—involving the AM, perirhinal face area, entorhinal cortex, and hippocampus—creates new associations between fresh faces and person identities. The second—involving neurons in the temporal pole—would grant quick, direct access to familiar face information. “Our findings might explain the rush of recognition that comes with seeing a familiar face,” Landi said. Notably, regions involved in both pathways are hit hard by Alzheimer's disease.
How many times must a face be seen, and in what context, before these fast-track temporal pole neurons encode it? This is one of many questions Landi, now a postdoc at the University of Washington in Seattle, is investigating. She also wants to know how this initial burst of recognition links up with other associations about the person behind the face. For example, person concept cells—dubbed “Jennifer Aniston neurons”—react to famous faces and even to familiar objects such as the Eiffel Tower (Quiroga et al., 2005). These cells react on a much slower timescale than the temporal pole neurons, and Landi wonders if the temporal pole neurons influence their activity.
How might these familiar face neurons relate to symptoms that surface in neurodegenerative disease? In people with frontotemporal dementia, prosopagnosia has been tied to atrophy in the right temporal lobe, particularly the medial temporal lobe, fusiform gyrus, and anterior temporal pole (Josephs et al., 2008; Erkoyun et al., 2020). People with AD also lose their ability to recognize people they know, a deficit ascribed to loss of both memory and visual perception (Mazzi et al., 2020). These different manifestations of facial recognition problems could reflect which parts of the brain are damaged in the two disorders, Landi said.
Landi’s study drew a distinction between the recognition of personally familiar faces versus those only viewed on a screen. Face-name recognition tests are widely used in Alzheimer’s research, but they tend to use famous faces as proxies for familiar ones, and hence might miss dysfunction of these temporal pole neurons, she said. To tap these cells, perhaps these tests could be personally tailored to include pictures of people a person knows in real life, she said.
Besides face cells, other specialized neurons such as hippocampal “place cells,” which are crucial for spatial memory, may also degenerate in AD (May 2008 news).—Jessica Shugart
References
News Citations
Paper Citations
- Moeller S, Freiwald WA, Tsao DY. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008 Jun 6;320(5881):1355-9. PubMed.
- Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010 Nov 5;330(6005):845-51. PubMed.
- Landi SM, Freiwald WA. Two areas for familiar face recognition in the primate brain. Science. 2017 Aug 11;357(6351):591-595. PubMed.
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005 Jun 23;435(7045):1102-7. PubMed.
- Josephs KA, Whitwell JL, Vemuri P, Senjem ML, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Petersen RC, Jack CR Jr. The anatomic correlate of prosopagnosia in semantic dementia. Neurology. 2008 Nov 11;71(20):1628-33. PubMed.
- Ulugut Erkoyun H, Groot C, Heilbron R, Nelissen A, van Rossum J, Jutten R, Koene T, van der Flier WM, Wattjes MP, Scheltens P, Ossenkoppele R, Barkhof F, Pijnenburg Y. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain. 2020 Sep 1;143(9):2831-2843. PubMed.
- Mazzi C, Massironi G, Sanchez-Lopez J, De Togni L, Savazzi S. Face Recognition Deficits in a Patient With Alzheimer's Disease: Amnesia or Agnosia? The Importance of Electrophysiological Markers for Differential Diagnosis. Front Aging Neurosci. 2020;12:580609. Epub 2020 Dec 21 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Landi SM, Viswanathan P, Serene S, Freiwald WA. A fast link between face perception and memory in the temporal pole. Science. 2021 Jul 30;373(6554):581-585. Epub 2021 Jul 1 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University College London
VU University Medical Center
In this interesting paper, Landi et al. provide a series of detailed investigations of macaque temporal pole and inferotemporal cell responses to faces and other stimuli. Despite cross-species differences, these findings might increase our understanding of face-recognition difficulties arising in human neurodegenerative diseases like the right-temporal variant of frontotemporal dementia (FTD) (Erkoyun et al., 2020) and posterior cortical atrophy (PCA, aka “visual-spatial Alzheimer’s disease”) (Graff-Radford et al., 2021).
The authors measured selectivity of macaque cell responses in right temporal pole and inferotemporal regions to both familiar and unfamiliar face, body, and object stimuli. Both temporal pole and inferotemporal cells responded selectively to face stimuli. However, while temporal pole cells responded more than three times more for familiar than unfamiliar monkey faces, there was no evidence of an effect of familiarity on inferotemporal cortex cell responses. Very high selectivity of temporal pole cells for familiar monkey faces was contrasted by similar inferotemporal responses for both monkey and human faces. Intriguingly, population response latencies were similar between temporal pole and inferotemporal cells. Given the lack of documented direct connections between temporal pole and inferotemporal regions in macaques, the authors propose the existence of two parallel pathways of face and person memory: one from inferotemporal to perirhinal and medial temporal regions, and another allowing rapid direct access to long-term semantic face information subserved by the temporal pole.
In the context of human neurodegenerative conditions, we see the following implications:
1. Apperceptive prosopagnosia (i.e., the inability to perceive and cognitively process the face) is a core feature of PCA and may be a defining feature of a proportion of individuals with ventral, visuoperceptual-predominant, PCA presentations (Crutch et al., 2017; Graff-Radford et al., 2021). Consistent with the apperceptive nature of face-perception difficulties, a ventral-clinico-radiological profile in PCA tends to comprise occipital and infero-temporoparietal rather than anterior-temporal atrophy (Groot et al., 2020). Whether the two proposed parallel pathways exist in humans raises the possibility of multiple routes to familiar-face recognition in PCA, with the hypothetical direct pathway proposed by Landi et al. possibly being relatively spared. However, it is worth noting that prominent low-level visual dysfunction arising in PCA may limit object identification regardless of stimulus type or category (Yong et al., 2014).
2. The authors suggest that their findings may relate to person-related agnosia following temporal-pole damage, carrying potential relevance to the right temporal variant of FTD. Documented radiological characteristics of right temporal variant include particular involvement of temporal poles extending to frontal and inferotemporal regions, emphasising right-sided predominant atrophy mirroring left-sided atrophy in semantic variant PPA (Erkoyun et al., 2020). Notably, initial prosopagnosia was more frequently observed in the right temporal variant of FTD (54 percent) versus semantic variant primary progressive aphasia (21 percent), behavioral variant FTD (4 percent) and typical (memory-predominant) Alzheimer’s disease (0 percent) (Abbate et al., 2019). It should be noted that although prosopagnosia at initial presentation is an uncommon feature of AD, it may arise in more advanced disease stages.
To conclude, we would like to reiterate that the current experiments were performed in macaques, hence detailed future investigation is essential to discover whether homologs of the proposed pathways also exist in human participants. Formal face-perception assessments are not routinely performed in a clinical/diagnostic setting, and these experiments are particularly challenging because both individuals with PCA or the right temporal variant of FTD have additional cognitive deficits (e.g., lower-level visual deficits in PCA) that may overlap with features of prosopagnosia. Furthermore, educational, cultural, and generational inter-individual differences may complicate the selection of appropriate stimuli (e.g., selecting famous or familiar faces). The ISTAART Atypical AD PIA is currently developing recommendations for assessing PCA features through a PCA working group, which will hopefully further facilitate the translation from basic neuroscience to clinical neuroscience studies.
References:
Ulugut Erkoyun H, Groot C, Heilbron R, Nelissen A, van Rossum J, Jutten R, Koene T, van der Flier WM, Wattjes MP, Scheltens P, Ossenkoppele R, Barkhof F, Pijnenburg Y. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain. 2020 Sep 1;143(9):2831-2843. PubMed.
Graff-Radford J, Yong KX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM, Jones DT, Murray ME. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021 Mar;20(3):222-234. PubMed.
Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, Dickerson BC, Vandenberghe R, Ahmed S, Bak TH, Boeve BF, Butler C, Cappa SF, Ceccaldi M, de Souza LC, Dubois B, Felician O, Galasko D, Graff-Radford J, Graff-Radford NR, Hof PR, Krolak-Salmon P, Lehmann M, Magnin E, Mendez MF, Nestor PJ, Onyike CU, Pelak VS, Pijnenburg Y, Primativo S, Rossor MN, Ryan NS, Scheltens P, Shakespeare TJ, Suárez González A, Tang-Wai DF, Yong KX, Carrillo M, Fox NC, Alzheimer's Association ISTAART Atypical Alzheimer's Disease and Associated Syndromes Professional Interest Area. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017 Aug;13(8):870-884. Epub 2017 Mar 2 PubMed.
Groot C, Yeo BT, Vogel JW, Zhang X, Sun N, Mormino EC, Pijnenburg YA, Miller BL, Rosen HJ, La Joie R, Barkhof F, Scheltens P, van der Flier WM, Rabinovici GD, Ossenkoppele R. Latent atrophy factors related to phenotypical variants of posterior cortical atrophy. Neurology. 2020 Sep 22;95(12):e1672-e1685. Epub 2020 Jul 16 PubMed.
Yong KX, Shakespeare TJ, Cash D, Henley SM, Nicholas JM, Ridgway GR, Golden HL, Warrington EK, Carton AM, Kaski D, Schott JM, Warren JD, Crutch SJ. Prominent effects and neural correlates of visual crowding in a neurodegenerative disease population. Brain. 2014 Dec;137(Pt 12):3284-99. Epub 2014 Oct 27 PubMed.
Abbate C, Trimarchi PD, Inglese S, Damanti S, Dolci GA, Ciccone S, Rossi PD, Mari D, Arosio B, Bagarolo R, Giunco F, Cesari M. Does the Right Focal Variant of Alzheimer's Disease Really Exist? A Literature Analysis. J Alzheimers Dis. 2019;71(2):405-420. PubMed.
Make a Comment
To make a comment you must login or register.