Anti-Tau Strategy PACs a Punch
Quick Links
In people with Alzheimer’s disease and other tauopathies, tau can accumulate at post-synapses in the dendrites of neurons. Could eliminating this tau temper neurodegeneration? In the May 26 Science Translational Medicine, researchers led by Natura Myeku, Columbia University, New York, reported that stimulating the postsynaptic G protein-coupled receptor (PAC1R) in a mouse model of tauopathy prompted the proteasome to digest tau. The treated mice performed better on memory tasks. Given that postsynaptic tau seeded more tangles in cultured neurons than did tau from pre-synapses, Myeku and colleagues believe that targeting PAC1R, and maybe other postsynaptic GPCRs, could become a treatment strategy.
- Postsynaptic tau from human and mouse brain seeds tangles in cultured neurons.
- Activating PAC1R boosts proteasome activity in mouse dendrites.
- As the proteasome clears tau, cognition improves.
As a postdoc with co-author Karen Duff, who is now at University College London, Myeku had previously shown that raising cyclic AMP levels boosted activity of protein kinase A (PKA), which then stimulated the proteasome. In 4-month-old rTg4510 mice, this cleared tangles and improved learning and memory (Dec 2015 news). But since cAMP is in almost every part of every cell, how to specifically ramp it up in the dendrites where tau accumulates?

PACking Up Tau. At the dendritic postsynapse, the neuropeptide PACAP activates the G protein-coupled receptor PAC1R. This kicks off a molecular cascade, wherein cyclic AMP activates protein kinase A (PKA), which in turn phosphorylates the 26S proteasome subunit. The activated proteasome then clears local tau. [Courtesy of Schaler et al., Science Translational Medicine, 2021.]
The researchers homed in on receptors primarily expressed at the post-synapses, such as PAC1R. First author Ari Schaler and colleagues now report that rTg4510 mice treated with the neuropeptide PACAP, which activates PAC1R, ramped up proteasome activity in dendrites and lowered tau in postsynaptic fractions of mouse cortical tissue. Treated mice better navigated a water maze than did untreated controls, and they better remembered objects placed into their cages. Alzforum first reported the finding when Duff presented at an EMBL Symposium held in Heidelberg, Germany (Jul 2017 conference news).
Now, the scientists report that tau accumulating in postsynapses may be particularly pathogenic. They took brain tissue from rTg4510 mice and isolated tau from pre- and postsynaptic fractions. In cell-based seeding assays, tangles bloomed when postsynaptic tau was added to cell medium, but not when presynaptic tau was used (see image below).
The same was true for extracts taken postmortem from cortical tissue of six people who had had AD. The postsynaptic fraction seeded more readily. “Postsynaptic compartments accumulate a lot of tau, and it seems to be more toxic. It’s a double whammy,” Myeku said.
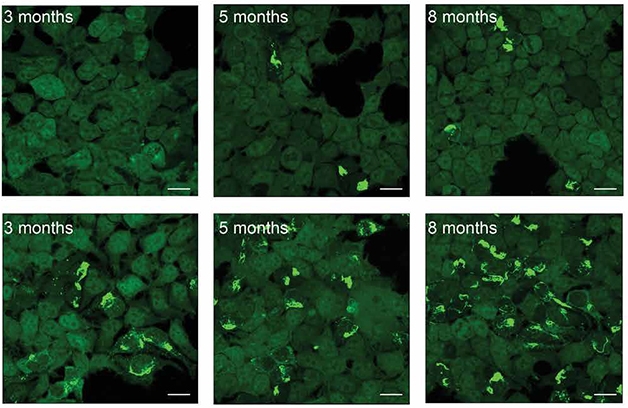
Post Is Worse. Tau taken from postsynapses of 3-, 5-, or 8-month-old tauopathy mice seeded more tangles in HEK cells (bottom) than tau taken from the presynaptic fraction (top). [Courtesy of Schaler et al., Science Translational Medicine, 2021.]
The findings hint that it may be better to target tau in the post-synapse than elsewhere. But how? Though PACAP itself slips through the blood-brain barrier and has even been reported to prevent cognitive decline in animal models of amyloidosis and Parkinson’s, it is quickly degraded in the blood and would have to be directly administered directly into the brain (Rat et al., 2011; Nonaka et al., 2012; de Souza et al., 2020). Myeku believes targeting PAC1R with a small molecule or compound more stable than the PACAP peptide might work, but recognizes the difficulty. “It may be challenging to target PAC1R with small molecules because of its large binding pocket,” she told Alzforum.
In the meantime, the researchers are exploring other GPCRs at the post-synapse. One is the 5-HT4 serotonin receptor. It activates the proteasome by stimulating cAMP and PKA, just as PAC1R does. The FDA has approved 5-HT4 agonists for other conditions, and Myeku is testing one, called prucalopride, in PS19 mice. Prucalopride improves gut motility and is prescribed for constipation. Other serotonin receptors have been targeted in AD, to no avail.—Chelsea Weidman Burke
References
Research Models Citations
News Citations
- Protecting Proteasomes from Toxic Tau Keeps Mice Sharp
- A New Explanation for Dendritic Tau: It’s Made There
Paper Citations
- Rat D, Schmitt U, Tippmann F, Dewachter I, Theunis C, Wieczerzak E, Postina R, Van Leuven F, Fahrenholz F, Kojro E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011 Sep;25(9):3208-18. PubMed.
- Nonaka N, Farr SA, Nakamachi T, Morley JE, Nakamura M, Shioda S, Banks WA. Intranasal administration of PACAP: Uptake by brain and regional brain targeting with cyclodextrins. Peptides. 2012 Aug;36(2):168-75. PubMed.
- de Souza FR, Ribeiro FM, d' Almeida Lima PM. Implications of VIP and PACAP in Parkinson's disease: what do we know so far?. Curr Med Chem. 2020 Mar 20; PubMed.
Further Reading
Primary Papers
- Schaler AW, Runyan AM, Clelland CL, Sydney EJ, Fowler SL, Figueroa HY, Shioda S, Santa-Maria I, Duff KE, Myeku N. PAC1 receptor-mediated clearance of tau in postsynaptic compartments attenuates tau pathology in mouse brain. Sci Transl Med. 2021 May 26;13(595) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
The University of Minnesota
Under physiological conditions, the microtubule-associated protein tau is enriched in axons but mostly devoid in postsynaptic structures such as the soma and dendrites, leading to a polarized cellular distribution of tau.
One common mechanism underlying multiple neurodegenerative diseases, including Alzheimer’s and frontotemporal dementia, involves the redistribution of tau from the axon into the somatodendritic compartments of neurons, leading to loss of tau polarity. The loss of polarity is followed by mislocalization of tau into dendritic spines, the postsynaptic structures found in most excitatory glutamatergic synapses, and by subsequent postsynaptic deficits. Although intensively studied, the cellular mechanisms by which pathogenic triggers, such as Aβ or disease-linked tau mutations, cause the postsynaptic accumulation of tau remain unknown.
In this elegant manuscript, Schaler et al. reported a novel PAC1 receptor-mediated signaling cascade that leads to the loss and/or reversal of tau polarity in animal models of frontotemporal dementia. They also reported that toxic postsynaptic tau species mediate the propagation to tau pathologies across cells. The study is of high translational significance because changes in tau polarity are one of the most commonly reported features in tauopathies, and the unraveled signaling cascade might be exploited to treat AD and other neurodegenerative diseases.
Mechanistically, it will be exciting to determine which step PAC1 receptors are involved in: tau redistribution to the somatodendritic domain, or subsequent tau mislocalization to dendritic spines.
University Clinic Cologne
This paper by Schaler et al. provides valuable insight into the contribution of postsynaptic pathological tau to disease progression in the rTg4510 mouse model. The authors, led by Natura Myeku, convincingly show that postsynaptic mutant tau is the main driver in synaptic and behavioral dysfunction, and that postsynaptic tau may be more pathological than presynaptic tau, which they demonstrate in a careful way in different assays. Notably, increased clearance of pathological tau, induced by PACAP-mediated increase in proteasomal degradation, markedly reduced disease severity.
This paper emphasizes that dendritic and postsynaptic tau may be the main driver of neuronal dysfunction in most tauopathies, and that increasing (postsynaptic) clearance may be beneficial in many disease paradigms.
This goes in line with our past findings clearly showing that tau mislocalization into the dendrites and dendritic spines (tau mis-sorting) is causative for neuronal dysfunction, and that sorting mechanisms usually targeting tau to the axons fail in disease paradigms of Alzheimer disease and related tauopathies (see e.g., Zempel et al., 2013; Zempel et al., 2017; Zempel and Mandelkow, 2014; Zempel and Mandelkow, 2019).
Schaler et al. provide an important proof of concept, yet the important limitation of this study is the huge overexpression of mutant tau in the rTg4510 mouse (more than 10x), and that only one isoform of tau is expressed. We recently confirmed that different isoforms of tau show a significantly different axodendritic distribution, and that their functional effect (in our case on cellular microtubules) may be different (Bachmann et al., 2021). In line with the data presented here, we also hypothesize that dendritic and postsynaptic tau drives the disease, but for tau to reach the dendrites, the sorting mechanism must fail early in disease.
Using iPSC- and SH-SY5Y cell-derived human neurons, we recently showed that successful targeting of tau to the axon may depend less than previously thought on the Axon Initial Segment (AIS), the neuronal polarity orchestrator in neurons (Bell et al., 2021). In combination with the data presented here by Schaler et al., it is fairly clear that apart from tau aggregates and modifications, aberrant tau localization should be considered a disease driver in future studies and therapeutic approaches.
References:
Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM. Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013 Nov 13;32(22):2920-37. PubMed.
Zempel H, Dennissen FJ, Kumar Y, Luedtke J, Biernat J, Mandelkow EM, Mandelkow E. Axodendritic sorting and pathological missorting of Tau are isoform-specific and determined by axon initial segment architecture. J Biol Chem. 2017 Jul 21;292(29):12192-12207. Epub 2017 May 23 PubMed.
Zempel H, Mandelkow E. Lost after translation: missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 2014 Dec;37(12):721-32. Epub 2014 Sep 12 PubMed.
Zempel H, Mandelkow E. Mechanisms of Axonal Sorting of Tau and Influence of the Axon Initial Segment on Tau Cell Polarity. Adv Exp Med Biol. 2019;1184:69-77. PubMed.
Bachmann S, Bell M, Klimek J, Zempel H. Differential Effects of the Six Human TAU Isoforms: Somatic Retention of 2N-TAU and Increased Microtubule Number Induced by 4R-TAU. Front Neurosci. 2021;15:643115. Epub 2021 May 25 PubMed.
Bell M, Bachmann S, Klimek J, Langerscheidt F, Zempel H. Axonal TAU Sorting Requires the C-terminus of TAU but is Independent of ANKG and TRIM46 Enrichment at the AIS. Neuroscience. 2021 May 1;461:155-171. Epub 2021 Feb 6 PubMed.
Make a Comment
To make a comment you must login or register.