Can Muscle Macrophages Coax Spinal Cord Microglia to Protect Neurons?
Quick Links
Can immune cells outside the central nervous system talk to microglia inside the brain? New findings from Séverine Boillée’s group at Sorbonne University, Paris, suggest they can—at least in mouse models of amyotrophic lateral sclerosis (ALS). Replacing diseased macrophages with healthy ones evoked protective responses from microglia, which preserved motor neurons, the authors report in the October 19 Nature Neuroscience. Although the myeloid cell switcheroo delayed symptoms and increased survival by only a few weeks, it suggests macrophages in peripheral tissue might influence disease processes inside the spinal cord. If true, figuring out how macrophages and microglia communicate may help researchers better understand ALS pathology.
- Peripheral macrophages communicate with CNS microglia, changing their gene expression.
- Wild-type macrophages calmed microglial inflammatory responses.
- Symptom onset was delayed and the mice lived slightly longer.
ALS arises from the slow deterioration of upper and lower motor neurons. Those in the spinal cord occupy a unique position, having cell bodies within the central nervous system (CNS) and axons extending out into muscles. As such, they can rub shoulders with two populations of myeloid cells: microglia in the CNS, and macrophages in the periphery (see the image below).
Microglia have been linked to motor neuron demise in ALS, but whether they play protective or harmful roles has been debated (Oct 2008 news; Geloso et al., 2017; Thonhoff et al., 2018). Macrophages have been found lurking around motor neuron axons before symptoms arise (Kano et al., 2012). Some studies suggest that they infiltrate the CNS and, once there, might conspire with microglia to cause neuroinflammation (Solomon et al., 2006; Butovsky et al., 2012). What if macrophages and microglia could talk to each other remotely?
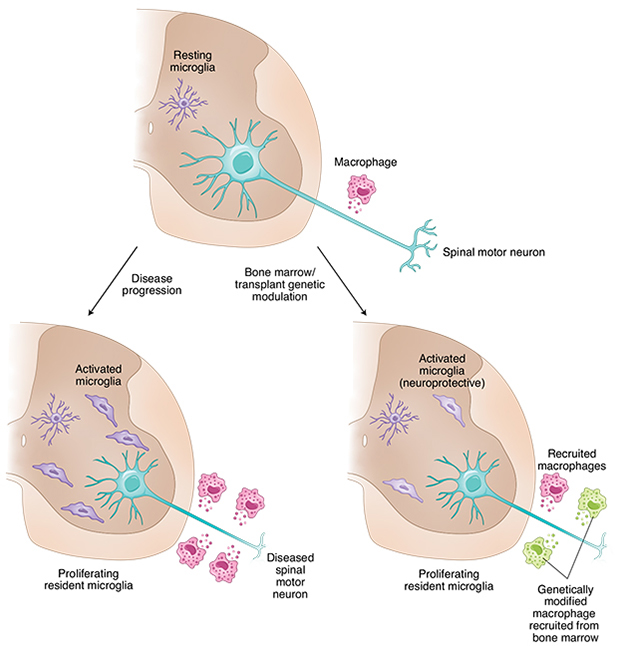
Myeloid Milieu. In the CNS (brown, top) spinal motor neurons deteriorate and microglia shape shift, as motor neuron disease progresses (bottom left). The role of peripheral macrophages in these processes is unclear, but modifications that make them less inflammatory seem to mollify brain microglia as well (bottom right). [Courtesy of Özdinler, 2020.]
Macrophage and Microglial Crosstalk
First author Aude Chiot and colleagues wanted to see how macrophages and microglia behaved in a rapidly progressing mouse model of motor neuron disease caused by expression of a human transgene carrying the G93A SOD1 mutation (Gurney et al., 1994). The mutant SOD is widely expressed in these animals, including in neurons and myeloid cells. Using RNA-sequencing, Chiot homed in on macrophage and microglia gene expression. To their surprise, these related cells had drastically different transcriptomes throughout the course of the disease. “We were quite stunned to see that microglia and macrophages were reacting completely differently,” Boillée said.
To see how wild-type macrophages would respond, Chiot ablated myeloid cells in approximately 50-day-old SOD1G93A mice using the chemotherapy drug busulfan. She then reconstituted the myeloid population four days later by transplanting bone marrow cells from wild-type mice. The wild-type macrophages were unable to slow microglial activation or disease progression. What if the macrophages were tweaked to make them less inflammatory?
Chiot again ablated the myeloid cells in the SOD1G93A mice, but this time she reconstituted them with cells from two different mouse strains—one that overexpressed wild-type SOD1, and one that had the NADPH oxidase gene knocked out. Nox2 generates reactive oxygen species and in macrophages helps drive inflammatory response, whereas SOD1, oddly enough, mops up said ROS. Again, Chiot tracked macrophage and microglial gene expression as the disease progressed.
In mice producing the SOD1 macrophages, microglia in the spinal cord began to express genes involved in a number of neuroprotective pathways, such as synaptogenesis signaling and oxidative phosphorylation, and they toned down inflammatory pathways, such as those involving TREM1signaling (see the image below). While this was transient, becoming less pronounced as the disease progressed, symptoms started two weeks later than in mice with macrophages carrying mutant SOD1, and the mice flushed with the new macrophages lived about two weeks longer as well. Mice repopulated with Nox2-negative macrophages had similarly reduced microglial activation, developed motor symptoms later, and lived slightly longer than SOD1G93A controls.

Inflammatory Pathways. Analysis of the top 25 immune cell trafficking pathways in microglia indicated that these cells ramped up inflammatory gene expression at early stage (middle column) and end-stage (right column) disease in SOD1G93A mice. However, swapping peripheral macrophages for those overexpressing wild-type SOD1 (left column) turned down inflammatory pathways (arrowheads) and reversed a synaptogenic one (arrow). [Courtesy of Chiot et al., Nature Neuroscience, 2020.]
Did the macrophages infiltrate the CNS to influence microglia directly? Boillée thinks not, because only 8 percent of the reconstituted myeloid cells found their way into the spinal cord. While this leakage is much less than seen when irradiation is used to ablate myeloid lineages in the periphery, some scientists still think it is too much to discount local effects on microglia.
If the macrophages do talk to microglia from outside the CNS, then how do they pull it off? That remains a mystery. “It is interesting that modulating the periphery gives rise to gene expression changes in the CNS,” Hande Özdinler, Northwestern University, Chicago, told Alzforum. She postulated several potential modes of communication, including exosomes, in a Nature Neuroscience editorial.
If true, this crosstalk might give scientists new opportunities to slow ALS progression. “Their findings suggest that peripheral macrophages, if modulated properly and at the right time, may have an impact on CNS microglia, turning them into a more ‘neuroprotective’ state and offering an overall therapeutic benefit for improving motor function in ALS,” wrote Özdinler. Boillée believe this might work, too. “We are now looking for the best pathways to target in the periphery that can alter the CNS,” said Boillée. “Then we could find molecules that work directly in the periphery to block that pathway.”
Boillée’s group is not the first to highlight the periphery’s role in ALS. Stanley Appel’s group at Houston Methodist Neurological Institute in Texas previously found that peripheral monocytes were activated in people with ALS (May 2017 news). “These results of Chiot et al. are not surprising, but rather confirming,” Appel told Alzforum (full comment below). “They suggest that axonopathy and neuromuscular junction impairment may be a very early event [in ALS] whereby macrophages may be signaled to become ‘bad guys.’ Replacing them with ‘good’ macrophages can offer a layer of protection for the axons.”
Shane Liddelow of New York University agreed. “There is a good body of literature suggesting that ALS does not originate as a CNS disease, but as an axonopathy,” he told Alzforum. “Something abnormal happens at the neuromuscular junction or the axon itself, which feeds back to the cell body and then into the CNS. These data fit into that fascinating hypothesis.”—Chelsea Weidman Burke
References
News Citations
- Microglia in ALS: Helpful, Harmful, or Neutral?
- Inside Out, or Outside In? ALS Turns on Monocytes in Blood
Paper Citations
- Geloso MC, Corvino V, Marchese E, Serrano A, Michetti F, D'Ambrosi N. The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front Aging Neurosci. 2017;9:242. Epub 2017 Jul 25 PubMed.
- Thonhoff JR, Simpson EP, Appel SH. Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr Opin Neurol. 2018 Oct;31(5):635-639. PubMed.
- Kano O, Beers DR, Henkel JS, Appel SH. Peripheral nerve inflammation in ALS mice: cause or consequence. Neurology. 2012 Mar 13;78(11):833-5. PubMed.
- Solomon JN, Lewis CA, Ajami B, Corbel SY, Rossi FM, Krieger C. Origin and distribution of bone marrow-derived cells in the central nervous system in a mouse model of amyotrophic lateral sclerosis. Glia. 2006 May;53(7):744-53. PubMed.
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012 Sep 4;122(9):3063-87. PubMed.
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772-5. PubMed.
Further Reading
Papers
- Liu Z, Cheng X, Zhong S, Zhang X, Liu C, Liu F, Zhao C. Peripheral and Central Nervous System Immune Response Crosstalk in Amyotrophic Lateral Sclerosis. Front Neurosci. 2020;14:575. Epub 2020 Jun 16 PubMed.
Primary Papers
- Chiot A, Zaïdi S, Iltis C, Ribon M, Berriat F, Schiaffino L, Jolly A, de la Grange P, Mallat M, Bohl D, Millecamps S, Seilhean D, Lobsiger CS, Boillée S. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival. Nat Neurosci. 2020 Nov;23(11):1339-1351. Epub 2020 Oct 19 PubMed.
- Özdinler PH. Help from peripheral macrophages in ALS?. Nat Neurosci. 2020 Nov;23(11):1311-1312. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Houston Methodist
These findings raise many questions. Are the peripheral macrophages affected prior to the CNS microglia being harmed? Is it a direct effect on the macrophages or are the macrophages indirectly affected by other cells, such as altered T regulatory cells or dendritic cells? The macrophages are certainly involved in the disease process, but they may not be the sole culprits and might be dictated to by something upstream. Upstream cells could be a variety of other immune cells that may be somewhat dysfunctional, and therefore, the macrophages can cause further difficulty. As for their effects on microglia, the macrophages might be talking with the neurons, rather than directly with the microglia. This is a neuronal-myeloid interaction, where both the macrophages and microglia influence neurons and other immune cells as well.
The results of this study are not surprising, but rather confirming. The predominant notion in ALS research is that ALS may start as an axonopathy where the axon dies back from the neuromuscular junction. However, a large group of researchers believes that it is not a bottom-up difficulty, it is a top-down one that starts from the cortex of the brain. This study is confirming because it suggests that axonopathy and neuromuscular junction impairment may be very early events whereby macrophages may be signaled to become “bad guys.” Replacing them with “good” macrophages can offer a layer of protection for the axons.
References:
Kano O, Beers DR, Henkel JS, Appel SH. Peripheral nerve inflammation in ALS mice: cause or consequence. Neurology. 2012 Mar 13;78(11):833-5. PubMed.
Sant Pau Biomedical Research Institute
In this study, Chiot and colleagues investigate the role of peripheral macrophages, located along peripheral motor neuron axons, and microglia, which surround the spinal motor neuron soma, in ALS. The authors use busulfan and transplantation of GFP+ bone marrow cells to replace peripheral macrophages with macrophages expressing less neurotoxic reactive oxygen species in SOD1 mouse models. Interestingly, their results elegantly show that this modulation, if performed at disease onset, reduces the activation of peripheral macrophages, but also of microglial cells, and extends survival.
One of the most interesting findings indicates that modifying the peripheral compartment may have an impact on the CNS and highlights the importance of studying peripheral immune cells.
Using unbiased technologies, such as single-cell RNA sequencing, to disentangle the heterogeneity of immune cells in the periphery and their gene expression profiles will certainly boost our understanding of the complex crosstalk between the peripheral and the central immune system. In this regard, it will be interesting to unravel how peripheral macrophages modulate microglial cells in the CNS, and if other cell types participate in this communication. A recent study suggested a role of natural killer cells on disease onset and progression in ALS and in the modulation of the microglial phenotype (Garofalo et al., 2020).
Finally, modulation of peripheral macrophages represents a new therapeutic strategy which might have implications in other neurodegenerative diseases. Importantly, these beneficial effects only occur when transplantation is performed at disease onset, which underscores the need of accurate biomarkers to diagnose ALS and enroll patients in clinical trials at early disease stages.
References:
Garofalo S, Cocozza G, Porzia A, Inghilleri M, Raspa M, Scavizzi F, Aronica E, Bernardini G, Peng L, Ransohoff RM, Santoni A, Limatola C. Natural killer cells modulate motor neuron-immune cell cross talk in models of Amyotrophic Lateral Sclerosis. Nat Commun. 2020 Apr 14;11(1):1773. PubMed.
University of Edinburgh and UK DRI
Like Séverine Boillée’s landmark observations in 2003, this elegant study once more highlights the therapeutic potential of myeloid cells in motor neuron disease.
It would be fascinating to find out how and which bone marrow- and yolk-sac-derived macrophages in peripheral nerves communicate with CNS microglia. Single-cell transcriptomic analysis and fate mapping may help to resolve this.
It appears from this study that the conditioning regimen for bone marrow transplantation plays a crucial role in attenuating the disease course in SOD1 mutant mice as the transplantation of wild-type bone marrow cells into irradiated mice conferred protection in previous studies, which was not the case with this busulfan protocol. The reduction in circulating white blood cells was also lower in irradiated than in busulfan-treated mice prior to transplantation, and important qualitative differences in compartmental myeloid cell reconstitution may exist.
Make a Comment
To make a comment you must login or register.