In DIAN-TU, Gantenerumab Brings Down Tau. By a Lot. Open Extension Planned
Quick Links
Against classical music and visual backdrops evoking Vienna, where the second AAT-AD/PD conference was to be held, the meeting instead unfolded Netflix-style. In this surreal age of COVID-19, the conference organizers dropped five days’ worth of prerecorded scientific programming all at once, tempting registrants around the world to binge-watch Alzheimer’s and Parkinson’s research symposia like so many episodes of “Stranger Things.” What stood out in this stream of slides, posters, and glimpses of speakers’ home offices? Some important news, actually. Consider this story of how gantenerumab gained a second wind in the Dominantly Inherited Alzheimer’s Network trials unit (DIAN-TU). While the primary outcome posted a null result, gantenerumab turned out to slash not only Aβ, but also tau. Could a higher dose work, after all?
Topline data presented by Randall Bateman, Washington University, St. Louis, suggest that the first two drug arms of the DIAN-TU trial platform—of Lilly’s solanezumab and Roche’s gantenerumab—were not a complete bust. Instead, the analyses finished to date point to nuanced effects of dose, time, disease stage, and biology. To be sure, the data shown at AAT-AD/PD did substantiate the earlier announcement that both therapeutic antibodies had fallen short on the trial’s primary endpoint, the DIAN-TU multivariate cognitive endpoint (Feb 2020 news). What happened? In short, symptomatic participants had descended into moderate dementia even before they could be titrated up to a high dose, whereas asymptomatic participants stayed stable throughout the trial regardless of whether they were on drug or placebo. This left the trial’s main question unanswered.
For solanezumab, a monoclonal antibody targeting soluble Aβ, this indeed marks the end of its exploration within DIAN, the global research network for families with autosomal-dominant Alzheimer’s disease. But all is not lost for gantenerumab, a monoclonal targeting aggregated forms of Aβ. Besides removing amyloid plaques from the brain and normalizing CSF Aβ42, this antibody reversed toward normal the elevated levels of CSF total tau and p-tau181, an AD-specific, pathological form of this neuronal protein. Gantenerumab further stemmed the rise of the general neurodegeneration marker CSF neurofilament light.
“I am very encouraged by the gantenerumab data. The tau and NfL response are good evidence that Aβ is driving these downstream changes,” Colin Masters of the University of Melbourne wrote to Alzforum.
“We are faced with the conundrum of a powerful drug that reaches target and has significant downstream effects, but no clinical effect, in an otherwise well-designed and well-executed trial,” Philip Scheltens of Vrije University Amsterdam summed up the data.
The effect sizes of this biomarker response were so large that they prompted the DIAN investigators and Roche to invite DIAN participants—who have devoted four to seven years of their lives to this trial, depending on when they enrolled—to join an open-label extension. It will explore high-dose gantenerumab therapy for several additional years.
Its goal? To see if sustained gantenerumab therapy near the highest tolerated dose removes both plaques and tangles all the way down to a hypothesized, yet-to-be-defined threshold at which cognition and function might start to benefit. The researchers also want to learn if a longer time on such a high dose gives the brain time to adjust to life without plaques and tangles—that is, to heal. Can a brain newly freed of this pathology cool its inflammation and perhaps restore synapses and circuits?
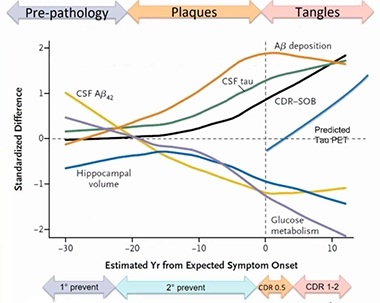
Staging DIAD. Twelve years—and counting—of observational research participation by families with pathogenic APP and presenilin mutations is continually refining this staging diagram of proteinopathy and downstream changes in this rare form of Alzheimer’s disease. [Courtesy of DIAN-TU.]
The design for this closely watched DIAN-TU-001 intervention trial was informed in large part by data gathered during the DIAN observational (Dian-obs) cohort study started by John Morris and colleagues at WashU, and now led by Bateman. In 2008, DIAN-obs began to characterize Alzheimer’s disease pathogenesis from early adulthood onward in carriers of rare pathogenic mutations in the APP and presenilin 1 and 2 genes, and compare it with their non-carrying relatives (Nov 2008 news). Clinical, cognitive, fluid biomarker, and brain-imaging measurements gathered since then helped build a model of disease progression. It aims to span a 40-year arc from early elevated CSF Aβ42 levels 30 years before a carrier develops symptoms, all the way to full-fledged dementia 10 years after onset (Bateman et al., 2012; McDade et al., 2018).
DIAN scientists used this observational data for two main purposes. They built a mathematical disease-progression model to serve as a quantitative framework for treatment trials in this form of AD. They also tap this observational data to supplement the placebo dataset of DIAN-TU treatment trials so that fewer mutation carriers have to be on placebo during a multiyear trial. (The odds of being on placebo deters people from committing to long clinical trials.)
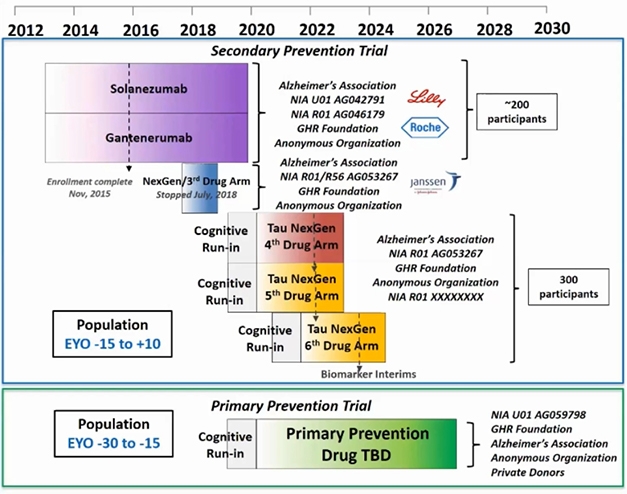
DIAN-TU Aims. Since 2012, the Dominantly Inherited Alzheimer’s Disease Trials Unit started dosing in three trials and completed two of them. A cognitive run-in phase is ongoing for two trials targeting tau and a primary prevention trial, whose study drugs are yet to be announced. [Courtesy of DIAN-TU.]
Preparations for an ongoing platform of successive DIAN-TU drug trials started nine years ago (Dec 2011 conference news). It engaged families the world over, together with pharma and other companies, academics and their site staff, funders, and other stakeholders, in an intensive public-private partnership. This PPP has thus far planned a series of seven drug arms, of which the solanezumab and gantenerumab treatment arms are the first two. The third arm, of the BACE inhibitor atabecestat, stopped in 2018 due to side effects, and arms four to seven will test drugs targeting tau and attempt primary prevention, respectively.
The solanezumab and gantenerumab trials began in December 2012 as a Phase 2 biomarker study and were later switched to a Phase 3 cognitive endpoint study. They enrolled across disease stages. This means they combined in one treatment group asymptomatic mutation carriers who could be as far as 15 years away from their estimated symptom onset and symptomatic people whose clinical dementia rating was 1 or lower at baseline. The first participant received the first of monthly infusions of antibody or placebo in March 2013; the last one got the final dose in November 2019. On average, the trial lasted 5.2 years. Because of the trial’s “common close” design, where everyone stays in until the last enrolled person gets his or her last dose, the earliest-enrolled completers were in this trial for a whopping 2,372 days, or 6.5 years.
Once Again: Too Little, Too Late?
Importantly, midway through this time period, the Alzheimer’s field at large realized that anti-Aβ antibodies need to be given at much higher doses than initially thought if they are to be effective, and that their ARIA side effect was more manageable than initially thought. As trialists did for the Aβ antibodies aducanumab and crenezumab, and for the A4 trial of solanezumab, DIAN-TU decided to ramp up. Starting in August 2016, they escalated the gantenerumab dose fivefold and, starting in September 2017, escalated solanezumab fourfold. This, however, came late in the trial, and symptomatic participants had already declined significantly.

Seven Years. Over its long course, the first trial in dominantly inherited AD switched from a biomarker to a cognitive outcome trial, and escalated doses of both study drugs. [Courtesy of DIAN-TU.]
The execution of the DIAN-TU-001 trial worked, despite widespread doubt early on whether a long, global intervention study could be done in this rare disease at all. Of 236 participants screened—from both within and outside of the pre-existing DIAN observation cohort—193 were randomized to double-blind treatment. Included in this number were 49 noncarriers randomized to placebo; they served as “cover” so no one would have to find out their mutation status. This generated treatment groups of 52 mutation carriers each for gantenerumab and solanezumab, and a pooled placebo group of 40 carriers.
Of those groups, 39, 36, and 30, respectively, completed the trial. This comes out to 7 percent dropout per year. Half of those left because their dementia had advanced beyond the point where they could deal with the infusions and assessments. “The overall dropout rate was amazingly low,” wrote Scheltens. Much shorter Alzheimer’s trials typically lose a third of their patients before the end. Remarkably, during the arduous regimen of monthly infusions plus periodic brain scans, cognitive tests, and fluid donation required, the participants’ compliance rate was 99 percent.
Alas, success on the trial’s main question was not to come. The randomization worked—i.e., the groups started out balanced across age, ApoE genotype, cognitive and clinical baseline, and other factors measured at baseline—but neither gantenerumab nor solanezumab met the primary, i.e., the DIAN-TU multivariate, endpoint. The difficulty was compounded by the realization, during analysis, that the trial data did not meet some of the assumptions previously made in the disease-progression model. Put simply, the data did not fit the model and, as a result, permitted no conclusions to be drawn about the effect of the high dose.
On the primary endpoint, neither treatment group did significantly better than placebo by year four, the last time point at which cognitive and biomarkers were both assessed. (At year five, cognitive but not biomarker data were assessed, and the groups were smaller due to the dropouts.) Overall, both placebo and drug arms declined.
Plotting each of the endpoint’s four component tests individually revealed that one test—logical memory—went against the three others. Participants improved on it year-on-year, showing an unexpected practice effect that was not apparent in the DIAN observational cohort data. The trial participants might have been learning because they took this test every six months, more frequently than it had been administered during the DIAN observation study, Bateman said. On two clinical measures shown, the CDR-SB and functional assessment scale (FAS), combined groups declined in nearly overlapping curves. DIAN-TU scientists are still analyzing additional outcome measures.
An aha! moment came when the scientists broke out the people who were presymptomatic at baseline from those who were symptomatic. The former improved greatly on the logical memory test, whereas the latter did not. On a second test, called Digit Symbol Substitution, presymptomatic participants also improved, but symptomatic people declined. This test was also performed more frequently in the trial than in DIAN-obs. And on the MMSE, which was given annually, presymptomatic people stayed largely stable, near 30, whereas the symptomatic ones slid from 26 to around 20. Overall, separating symptomatic and presymptomatic participants revealed, at a glance, a stark difference between them on all tests used. Symptomatic participants started off much worse and declined; asymptomatic participants started nearer the tests’ ceiling or normal range, and stayed there.
“I think mixing the asymptomatic with the symptomatic effectively cut the power of the study by half. The numbers of cases were too small to allow for a workable cognitive readout,” Masters wrote to Alzforum. On the CDR-SB and FAS, too, all symptomatic groups declined and all presymptomatic groups stayed stable.
To Scheltens’ mind, the absence of evidence on the cognitive endpoint in this trial is not evidence of absence. He thinks that treated participants in this trial might have had slight shifts in cognition, or indeed function, that went undetected. Scheltens suspects the tests used were too “old school” to pick up subtle changes in this relatively young population even if they were sensitive to decline in the observational cohort. He hopes the DIAN-TU-001 data can be mined to look for those shifts. “I always think of the adage, ‘every trial is as good as its outcome measure,’” Scheltens wrote to Alzforum (full comment below).
Part of the problem with the trial data arises from the disease-progression model. It constitutes the statistical construct for analysis of the primary endpoint data in both trial arms. The model was fitted to the DIAN-obs data. To compensate for limitations inherent in running treatment trials in this rare disease, the model made assumptions, for example that performance on all tests would decline, or that variance would be constant across presymptomatic and symptomatic participants, and also about when symptoms would likely start.
“However, and unfortunately, our trial data did not match all assumptions of the model,” Bateman said. The placebo group improved on some tests; measures did not change longitudinally the way cross-sectional analysis had suggested, and asymptomatic and symptomatic carriers had different degrees of variance on the MMSE.
On safety, the news was good. Side effects in the DIAN-TU-001 trial were as in prior trials with these antibodies. Nothing new cropped up. Solanezumab did not cause ARIA. Gantenerumab brought with it more ARIA-E and ARIA-H seen on MRI. Most instances of ARIA resolved spontaneously and were asymptomatic, or they were mildly symptomatic such that the symptoms came up only once participants were specifically asked. “We were very pleased with the great tolerability of gantenerumab in these subjects. It raises the question of whether we could have gone higher with the dose,” Rachelle Doody of Roche said in an accompanying discussion.
Biomarkers to the Rescue?
The U-turn toward hope came with the biomarker analysis. The near-total completion rate of assessments in this trial generated a plethora of fluid and imaging data. At AAT-AD/PD, Bateman first presented prespecified analyses of each drug arm compared with placebo. As was expected, gantenerumab showed target engagement via a statistically significant reduction on PiB PET. At 0.64 SUVR, this reduction was large, and similar to the reduction previously reported for high-dose gantenerumab in a LOAD open-label extension (Dec 2019 conference news).
On downstream disease-modification markers, FDG PET and thickness of the precuneus, an early affected part of the cortex, were not significantly different in the combined asymptomatic/symptomatic groups with either antibody. Bateman did not report tau PET data at AAT-AD/PD because DIAN-TU added this marker midway through the trial, hence has few scans taken at baseline for comparison.
In CSF, the Aβ42/40 ratio was up significantly, and CSF total tau and CSF pTau181 were down by nearly a third. These three changes all indicate a reversal toward normal levels, and came in at p values of below 0.001. CSF neurofilament light chain (NfL) showed less increase on gantenerumab than placebo, at p=0.024.
How about solanezumab? It also engaged its primary target, as evidenced by a steep increase of total CSF Aβ42; however, of the downstream markers, only CSF NfL was significantly different between drug and placebo, and its change pointed toward worsening on drug, with p=0.017. CSF total tau and pTau181 did not budge.
To show how gantenerumab engaged its target over time, Bateman showed PiB PET scans taken at baseline and at one, two, three, and four years. For presymptomatic and symptomatic carriers grouped together, amyloid burden diverged at one and two years and, once participants went on the high dose, the gap widened. By year four, the effect size of amyloid reduction, expressed as percent relative risk, was large—209 percent reversal toward normal.
As was the case for the cognitive data, breaking open the PiB data by presymptomatic and symptomatic groups revealed just how different they were at the start of the trial. Unlike for the cognitive readouts, however, both presymptomatic and symptomatic participants responded to gantenerumab with amyloid reduction. Expressed in centiloids, presymptomatic carriers started out near 30; those on placebo added some 10 centiloids, whereas those gantenerumab lost 10, for a difference of 20 centiloids. Symptomatic carriers started out around 85 centiloids; placebo recipients went up above 100 and gantenerumab recipients down to about 65. Mutation noncarriers posted zero centiloids throughout the trial.
“When you look at the PET Aβ loads in these two groups, you see a large baseline difference, which affects cognition more than biomarkers in terms of response to intervention. The lowering effect on Aβ load is very convincing, but not enough to expect a cognitive benefit. I would have expected to see a cognitive benefit if they had got it down to 30 to 40 centiloids,” Masters wrote to Alzforum.
The Aβ42/40 ratio, which reflects active deposition, returned toward normal on gantenerumab and worsened on placebo, Bateman reported.
The bigger surprise was the strength of the data on downstream markers. At AAT-AD/PD, Bateman showed that both tau measures reversed direction toward normal in people on gantenerumab, posting at least 30 percent difference between drug and placebo by year four. In people on placebo, both total tau and p-tau181 rose year-on-year from baseline; on drug, both decreased year-on-year. In noncarriers, total and p-181 tau stayed unchanged. Commentators agreed that these changes are large and likely biologically significant. Neurofilament light chain, considered a generic indicator of active neurodegeneration, rose more in carriers on placebo than on gantenerumab but stayed largely stable in noncarriers, for a group difference of 10.8 percent. Additional downstream markers remain to be analyzed for differences by disease stage.
Because gantenerumab engaged its target and moved several downstream markers of Alzheimer’s disease, DIAN-TU and Roche are inviting all members of this trial—regardless of whether they had been on gantenerumab, solanezumab, or placebo—into an open-label extension study. The OLE is currently approved for three years. Bateman hopes that if continued biological improvements are seen, with trends toward clinical and cognitive benefit, it will run longer.
This OLE will be exploratory, i.e., have no placebo group. But it will offer the participants access to high-dose gantenerumab. Bateman told Alzforum that mutation carriers will be titrated up to a goal of 1,020 mg per infusion, the dose in Roche’s ongoing Phase 3 GRADUATE program for mild late-onset AD. Tolerability and ARIA allowing, the dose might go even higher. “Due to the late increase in dose, we did not completely normalize amyloid load in the groups,” Bateman said. “I believe in the OLE, they will get there,” Masters said.
What’s to be gained? Some answers, and perhaps some clinical benefit, after all. Will high-dose gantenerumab reduce amyloid plaques in these DIAD families below the threshold of positivity? If so, will downstream markers of AD return to normal, as well? And will that help? In other words, will some symptomatic carriers stabilize or get better? And will the presymptomatic people continue to stay well?
DIAN-TU researchers can compare results from this OLE to disease progression as observed in the DIAN-obs cohort. Doody emphasized that because this OLE is not a new trial, it will not yield definitive answers. But even so, it can contribute important information to the package of converging evidence from multiple sources that will ultimately decide gantenerumab’s future as an Alzheimer’s treatment.
Besides tracking cognition, function, and the biomarkers that were part of the blinded DIAN-TU trial, the OLE can add markers to assess possible effects downstream of amyloid reduction. This could include, for example, CSF sTREM2, YKL-40 and PET markers of glial activation, neurogranin and diffusion tensor imaging or other markers of neurodegeneration, and SV2A, NPTX2, or other markers of synaptic health.
After years of infusions, and travel to clinics for scans, tests, and needle pricks—all for a seemingly null result—do the DIAN participants want to continue? Some among them have given five years of their lives to a placebo, and this will be their first chance to get a drug that moves amyloid and tau. For the open-label extension, all participants need to find out their mutation status, as it would be unethical to expose noncarriers to an investigative medicine. In private, DIAN participants debate this question with some anguish.
And yet, they are stepping up again. In force. “The U.K. participants are very interested. They see this OLE as a logical progression and are willing to find out their mutation status to go on it,” Catherine Mummery of University College London wrote to Alzforum. “Our DIAN-TU participants were very disappointed when the trial was pronounced ‘negative,’ but are hopeful about this recent news and happy for the possibility of participating in the OLE,” wrote Bill Klunk from the University of Pittsburgh School of Medicine. The same feedback came from other sites in in the United States and other countries. Said Bill Brooks of Neuroscience Research Australia, who leads the DIAN-TU site in Sydney, “We plan to begin the OLE as soon as the COVID-19 situation allows.”—Gabrielle Strobel
References
Therapeutics Citations
News Citations
- Topline Result for First DIAN-TU Clinical Trial: Negative on Primary
- DIAN: Registry for eFAD to Chart Alzheimer’s Preclinical Decade
- DIAN Forms Pharma Consortium, Submits Treatment Trial Grant
- Confused About the DIAN-TU Trial Data? Experts Discuss
- Amyloid Clearance: Check. Cognitive Benefit: Um … Maybe.
Paper Citations
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012 Aug 30;367(9):795-804. PubMed.
- McDade E, Wang G, Gordon BA, Hassenstab J, Benzinger TL, Buckles V, Fagan AM, Holtzman DM, Cairns NJ, Goate AM, Marcus DS, Morris JC, Paumier K, Xiong C, Allegri R, Berman SB, Klunk W, Noble J, Ringman J, Ghetti B, Farlow M, Sperling RA, Chhatwal J, Salloway S, Graff-Radford NR, Schofield PR, Masters C, Rossor MN, Fox NC, Levin J, Jucker M, Bateman RJ, Dominantly Inherited Alzheimer Network. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology. 2018 Oct 2;91(14):e1295-e1306. Epub 2018 Sep 14 PubMed.
Other Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
Q&A with Randall Bateman of Washington University School of Medicine
Q: Compared with February, how are you feeling now about this trial?
A: I am excited. We are on the right track in terms of impacting the biology of the disease. We couldn’t make a conclusion on cognitive measures because we increased doses later and asymptomatic participants didn’t decline. I’m looking forward to the gantenerumab exploratory OLE to continue to inform about what this mechanism can do.
Q Is there a simple way to explain the problem with the model?
A: It’s complicated Bayesian statistics. The bottom line is the data that came from the trial did not match several of the assumptions in the model. We are working on dealing with those assumptions, but do not think it changes the cognitive outcomes of this trial.
Q: With the benefit of hindsight, what would you change?
A: Use maximal dose at the outset of the trial. Enroll more participants for the asymptomatic population. Follow asymptomatic participants longer, which we are doing in the exploratory OLE. Choose cognitive outcomes based on their trial performance, which we can do now. Read out biological signals earlier to decide on cognitive endpoint studies, which we are doing in the Tau NexGen studies.
Q: You showed CDR-SB, FAS, and biomarkers. What about all the other secondary outcome measures? Were there no signals with gantenerumab on any of them? Or did you not want to cherry-pick the “good” ones in this presentation?
A: We haven’t fully analyzed the other measures yet, but most are consistent with the main findings reported. It would be a mistake to pick out analyses just because they showed a benefit.
Q: On the clinical/functional measures you showed, it looks as if the symptomatic people on gantenerumab were trending a little better?
A: Not statistically significant. We don’t want to cherry-pick positive trends without careful preplanned analyses.
Q: When you split biomarker outcomes by symptomatics versus asymptomatics, was there no increased activity in precuneus in the asymptomatics over placebo after four years on gantenerumab? Precuneus is an early affected area.
A: This still needs to be analyzed.
Q: Considering when in the trial the dose went up, it seems you did not measure much of the effect the high dose might have been having on biomarkers because it would have happened after year four. Is that information lost, or could it still be gathered?
A: We will be able to pick up the high-dose exposures after year four for those participants who enter the exploratory OLE, as they will complete a full biomarker battery before resuming or starting gantenerumab.
Q: Why did solanezumab apparently make things worse?
A: For some measures of cognition and NfL in the DIAN-TU trial, the solanezumab arm didn’t do as well as placebo, but it’s not clear to me why. It could be due to the small numbers, that although they appeared well-balanced, something about the solanezumab group was naturally going to progress faster. Solanezumab has an enormous amount of safety data and trend toward benefit in sporadic mild AD, so that reassures me this may not be a solanezumab-specific effect. There are also differences in the population of age, mutation status, and stage of disease being tested, so it’s possible those factors have an effect.
Q: In the OLE, will you add additional markers to track the gantenerumab effect in a more granular way?
A: We are already adding additional biomarkers to the completed trial, because we have established an extensive biomarker bank of CSF and blood samples. We do have plans to analyze extensive biomarkers in existing and future samples. Much more to come …
Q: Why wasn’t the amyloid plaque reduction in symptomatics even larger? In the ScarletRoAD/MargueriteRoAD gantenerumab OLE, amyloid dipped below the positivity line. What’s going on here? (Mutation-driven overproduction overpowered gantenerumab effect?)
A: We are getting the data and analyzing it in a PK-PD model to help address this question. There was a large effect (209 percent) in the symptomatic group, and we are considering if longer or higher dosing could lower this more or faster.
Q: Are the symptomatic participants invited into the OLE? Why? You know it will not help them—unless you think dose was too low and more amyloid reduction could still help them.
A: For at least two reasons: 1) They may have received too low a dose, so we don’t know what high dose will do. There are other examples of amyloid antibodies, where dose in the symptomatic stage seems to be critical to see a clinical benefit. This is why the OLE will start with high dose. 2) Symptomatic participants will provide extensive insight into the biology and biomarker changes that come with removing amyloid plaques.
Q: Will you compare OLE participants with DIAN-obs “placebos”?
A: Yes, and we also will use placebo data in past and future/concurrent DIAN-TU trials.
Q: What do these data mean for the GRADUATE trials? They enroll symptomatics up to CDR 1.0/MMSE 22. That’s the disease stage at which gantenerumab did not work in DIAN-TU. Would not your data now suggest a biomarker response but no cog benefit?
A: No. We didn’t treat with the high-dose gantenerumab uses in the GRADUATE studies in the early/mild AD clinical dementia stage. We started with low-dose gantenerumab in early symptomatic range of CDR 0.5 and 1, but by the time dose was increased to high dose, many participants had advanced to more severe stages. I do think the GRADUATE studies will address the potential for high-dose gantenerumab symptomatic clinical benefit in sporadic AD.
Q: Why not be more granular in your analysis than just symptomatic versus asymptomatic groups? Isn’t someone who is one year away from EYO different than someone at minus 10? Why not spaghetti plots for each participant for such a small trial?
A: We are working on it. We have reviewed this data, but can’t show individual plots easily due to unblinding to both drug and mutation status.
Q: It seems prevention of dementia in asymptomatic people with gantenerumab is still alive.
A: Very much alive.
Alzheimer Center Amsterdam; Head EQT Life Sciences Dementia Fund
The virtual presentation of the DIAN-TU results at AD/PD was skillfully done and rich in content. The clinical endpoint was negative, as was reported before.
Especially striking was the decline in the symptomatic group with CDR>0 equally in all three arms, but not in all four tests. The logical-memory test seemed to suffer from a learning effect, while the MMSE, ISLT, and DSST behaved as expected, sadly without a treatment effect in either arm.
Of note was the absence of any decline in the CDR=0 group over such a long period (average trial duration 5.2 years). As indicated in my previous comment in February, I was very keen to see the biomarker results. I had higher hopes for gantenerumab in this respect than for solanezumab based on the mode of action. And here, I was not disappointed at all. In the gantenerumab group (n=39) at year four of the trial, the mean corrected SUVr was significantly down, with 0.641; this was not significant for solanezumab.
CSF Aβ 42 (+42.1 percent), NfL (10.8 percent), total tau (-32.2 percent) and p-tau 181 (-30.6 percent) were all significantly changed, compared to mutation carriers on placebo, almost returning to normal levels! For solanezumab this was only (and less) true for Aβ42; for NfL the values actually increased in the active group, indicating more neuronal damage than with placebo. The target engagement of gantenerumab was impressively shown by a -29 percent centiloid change at year four, while the curves already started to separate at year one. I would be curious to see plasma tau levels over the four years, as well.
Of note, the occurrence of ARIA in the gantenerumab arm was as expected: 25 percent for new hemorrhages as compared to 10 percent in the placebo arm and 19.2 percent for ARIA-E as compared to 2.5 percent on placebo. All without clinical meaningful sequelae or dropout. Overall dropout rate was amazingly low with a mean 7 percent per year, mainly due to decline in the mutation carrier group.
So, here we are faced with the conundrum of a powerful drug that reaches target and has significant downstream effects, but no clinical effect, in an otherwise well-designed and well-executed trial.
I always think of the adage: “every trial is as good as its outcome measure.” The fact that no effect was measured does not mean there was no effect at all. It may mean we need to be more creative and sensitive. In this relatively young and unique population, “old school” measures like MMSE, DSST and ISLT may not respond to subtle changes taking place in the brains of relatively mildly affected individuals, although they did track the decline over time. In the asymptomatic individuals, ceiling effects may have hindered outcome as well. The experience with the logical-memory test also teaches us that learning effects may seriously influence test outcomes. It may well be that functional improvements have been there but were not adequately measured. Combining functional and cognitive measures showed greater sensitivity to change in a very mildly affected cohort (Jutten et al., in press).
What did the caregivers observe? And what did the patients themselves observe? Was there an effect on any other measures (I did not see CDR figures)? There must be data to be mined to look for subtle differences.
For the future, using wearables, and remote sensitive cognitive testing using mobile devices—as I believe is planned in the next phase of the DIAN-TU program—may also help to understand the effects of these interventions. For sure we need ultrasensitive measures that tap into real-life issues, without ceiling effects, especially if interventions are started in the asymptomatic phase.
From the biomarker data as presented at AD/PD, I fully understand and endorse that the participants are offered open-label treatment with gantenerumab to learn more about the long-term effects and to offer them the possible benefit of changing the biology of their fatal disease.
References:
Jutten RJ, Harrison JE, Brunner AJ, Vreeswijk R, van Deelen RA, de Jong FJ, Opmeer EM, Ritchie CW, Aleman A, Scheltens P, Sikkes SA. The Cognitive-Functional Composite is sensitive to clinical progression in early dementia: Longitudinal findings from the Catch-Cog study cohort. Alzheimers Dement (N Y). 2020;6(1):e12020. Epub 2020 Apr 17 PubMed.
University of Southern California Keck School of Medicine
Despite the null cognitive effects after four years of randomized treatment to one of two Aβ antibodies or placebo, DIAN TU substantially advanced trial methods by demonstrating the feasibility of an international network of sites, establishing a trials platform upon which an overlapping series of drugs could be tested; and then developing a disease-progression model, an efficient cognitive composite scale, and methods to observe biomarkers over the long term.
It’s important to consider that the trial was not originally intended as an efficacy trial and because of the small sample size any statistically significant positive-outcome scenario most likely would have led to considerable uncertainty and controversy. The platform demonstrated its resilience when a third drug, atabecestat, was introduced and quickly withdrawn because of toxicity, and when the ongoing cognitive run-in arm was added in its place. The neuropsychological composite is efficiently composed of just a few tests including story recall, a word list, digit symbol substitution, and the persistently versatile MMSE that appears as a component of several other composites. Although the assumptions of the multivariate disease progression model weren’t met, the current outcomes over four to seven years provide otherwise unobtainable data to improve modeling disease progression and multivariate biomarker change. The opportunity to track “traditional” and new biomarkers with gantenerumab over perhaps six to nine years, or until the end of the Phase 3 trials in sporadic AD, may provide for a more sophisticated understanding of clinical illness progression than we might otherwise achieve.
Brown University
We were disappointed that the DIAN-TU trial did not meet its primary cognitive endpoint. The small sample that combined preclinical and symptomatic patients, variable range in expected time to disease onset, and late escalation to the high dose may have contributed. Some assumptions of the model and data were also not met.
It’s encouraging that gantenerumab achieved its original biomarker goal showing a substantial reduction in amyloid PET and expected directional changes in CSF amyloid, phospho-tau, and neurofilament light. The open-label extension will further explore the biomarker effects and their relationship to disease progression. Participants on a recent DIAN family webinar expressed strong interest in continuing on in the exploratory open label extension.
Disclosure: Dr. Salloway is the Project Arm Leader for gantenerumab in the DIAN-TU trial and he receives research support from and is a consultant to Roche and Eli Lilly.
Washington University in St. Louis
The biomarker effects in the gantenerumab arm are highly promising. In particular the robust effect on CSF measures of tau is a key point as the OLE moves forward. As a field we still do not understand the relationship between CSF measures of tau and the neurofibrillary tau tangles thought to be measured by the PET radioligands. Seeing if a reduction in CSF tau levels also corresponds to changes seen using tau PET is critical as trials move toward incorporating anti-tau therapies. The DIAN-TU OLE should be able to answer this question.
Make a Comment
To make a comment you must login or register.