ZFAND1 Triggers Proteasomal Clearance of Stress Granules
Quick Links
The misfolding of some proteins that cause neurodegenerative disease has been linked to liquid-liquid phase separation and membraneless organelles called stress granules. New findings suggest that the proteasome helps to dissolve these organelles and may act as the first line of defense against certain protein aggregates. In the June 7 Molecular Cell, researchers led by Alexander Buchberger at the University of Würzburg, Germany, identified a protein, ZFAND1, that recruits the 26S proteasome to stress granules. Without ZFAND1, these buffers against cellular stress accumulated greater loads of damaged protein and persisted longer in the cell. The aberrant granules were eventually removed by autophagy.
- ZFAND1 helps recruit proteasome to stress granules.
- Without ZFAND1, cells are slow to clear granules induced by arsenite.
- Proteasomal clearance may be first line of defense against this type of protein aggregate.
“A key finding of our paper is that the 26S proteasome plays an important role in normal clearance of stress granules,” Buchberger told Alzforum. “These data emphasize that we need to better understand how stress granules are formed and cleared.” In future work, he plans to dissect what distinguishes normal, transient stress granules from aberrant, pathological ones.
“We have known that VCP [valosin-containing protein] mediates stress granule removal, but the mechanics of this are poorly understood. This manuscript highlights the role of a protein that appears to function like a VCP adapter protein,” Ben Wolozin at Boston University wrote to Alzforum (see comment below).
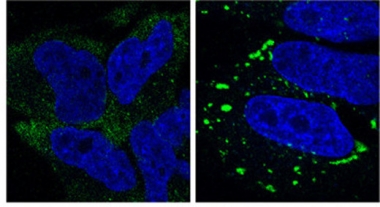
Persistent Stress.
Stress granules (green) linger in cells lacking ZFAND1 (right), whereas control cells (left) clear them within two hours. Nuclei are blue. [Courtesy of Molecular Cell, Turakhiya et al.]
Why do stress granules form in the first place? Scientists believe that during times of stress, cells pause protein production to save energy for more immediate responses. They shunt untranslated mRNA, proteins, and ribosomes into cytoplasmic granules, which dissipate once stress is relieved (for review see Panas et al., 2016; Protter and Parker, 2016). It is unclear exactly how they disassemble, but researchers have identified several proteins that localize to stress granules and thus might play a role. These include the 26S proteasome, ZFAND1, and VCP. The latter, an ATPase that helps separate out proteins tagged with ubiquitin for proteasomal disposal, is genetically linked to amyotrophic lateral sclerosis and frontotemporal dementia, implicating stress granule buildup in these diseases (Dec 2010 news; Jun 2017 news).
Buchberger and colleagues set out to define ZFAND1’s role in stress granules. First author Ankit Turakhiya confirmed, through pulldown and co-immunoprecipitation experiments, that ZFAND1directly binds the 26S proteasome, but found no binding to VCP. When Turakhiya exposed HeLa cells to arsenite, a commonly used trigger of stress granules, ZFAND1 appeared in these new organelles. Under these conditions, ZFAND1 bound the proteasome more strongly than before, and gathered in VCP as well. Additional experiments with truncated ZFAND1 mutants demonstrated that its N-terminus interacted with the 26S proteasome, while its C-terminus attached to VCP and to stress granules. ZFAND1 was able to bind to stress granules in the absence of VCP, suggesting it arrives first at the scene and then recruits the proteasome and VCP.
The authors knocked out the protein in HeLa cells and then treated them with arsenite. Stress granules formed normally, but had not dissolved by two hours after arsenite washout, as they did in control cells (see image above). In the absence of ZFAND1, neither VCP nor the 26S proteasome localized to stress granules, confirming the recruitment hypothesis. Adding back wild-type ZFAND1 rescued recruitment of VCP and the proteasome as well as clearance of stress granules, whereas adding mutant ZFAND1 lacking either the N-terminal or C-terminal domains did not. The results suggest that ZFAND1 and the proteasome are required to dissolve these granules.
In keeping with this, defective ribosomal products, a.k.a. DRiPs, glommed onto stress granules in the knockout cells. DRiPs consist of incomplete, misfolded polypeptides that become ubiquitylated. Their presence denotes aberrant, long-lasting stress granules (Seguin et al., 2014; Ganassi et al., 2016). In control HeLa cells, stress granules and DRiPs appeared after arsenite stress, but did not associate with each other, and both were gone after two hours of recovery. A proteasomal inhibitor caused DRiPs to accumulate in stress granules after arsenite treatment, however, mimicking the effects of ZFAND1 knockout. This supports the idea that ZFAND1 promotes proteasomal clearance of these damaged proteins.
As a last line of defense, aberrant stress granules can be cleared by autophagy (Mateju et al., 2017). Consistent with this, an autophagy inhibitor had no effect on stress granule clearance in control cells, but prevented their eventual clearance in ZFAND1 knockouts.
Buchberger noted that ZFAND1’s role seems to be limited to the arsenite type of stress, however. Stress granules induced by heat, osmotic, or oxidative stress were cleared normally in ZFAND1 knockout cells. “This suggests that different types of stress granule might have different compositions and clearance pathways,” Buchberger told Alzforum.
Other researchers were intrigued. “These findings suggests considerable specificity in the molecular mechanisms underlying stress granule disassembly. Delineation of such diverse, stressor-dependent responses will likely be crucial in guiding the development of tailored therapeutic strategies across the spectrum of neurodegenerative disorders,” Rickie Patani at the Francis Crick Institute, London, wrote to Alzforum.
How might the findings relate to pathological protein accumulation in neurodegenerative disease? Arsenite causes acute protein misfolding, whereas neurons build up misfolded proteins over decades, Buchberger noted. Aberrant stress granules in neurons have been found to contain pathological TDP-43, FUS, and tau (Jul 2010 news; Jul 2010 news; Jun 2012 news). They are associated with neurodegenerative disease, particularly ALS and FTD (Aug 2017 news). Perhaps aberrant stress granules act as seeds for pathogenic fibrillar protein aggregates, Buchberger suggested.
Buchberger believes it would be worth checking for ZFAND1 mutations in patients with neurodegenerative disease who do not carry a known mutation. He suspects ZFAND1 variants would have a milder effect than VCP mutations. This is because VCP also participates in autophagy, so defective VCP would impair both clearance pathways.—Madolyn Bowman Rogers
References
News Citations
- Adding ALS to the Manifestations of VCP Mutations
- Familial ALS Linked to Both Neuron and Astrocyte Pathology
- Honolulu: TDP-43 Gets a Place in the Sun
- Going Nuclear: First Function for FUS Mutants
- Paper Alert: Stress Granules Get Tangled Up in Tau
- Newest ALS/FTD Gene Keeps Spotlight on Stress Granules
Paper Citations
- Panas MD, Ivanov P, Anderson P. Mechanistic insights into mammalian stress granule dynamics. J Cell Biol. 2016 Nov 7;215(3):313-323. PubMed.
- Protter DS, Parker R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016 Sep;26(9):668-79. Epub 2016 Jun 9 PubMed.
- Seguin SJ, Morelli FF, Vinet J, Amore D, De Biasi S, Poletti A, Rubinsztein DC, Carra S. Inhibition of autophagy, lysosome and VCP function impairs stress granule assembly. Cell Death Differ. 2014 Dec;21(12):1838-51. Epub 2014 Jul 18 PubMed.
- Ganassi M, Mateju D, Bigi I, Mediani L, Poser I, Lee HO, Seguin SJ, Morelli FF, Vinet J, Leo G, Pansarasa O, Cereda C, Poletti A, Alberti S, Carra S. A Surveillance Function of the HSPB8-BAG3-HSP70 Chaperone Complex Ensures Stress Granule Integrity and Dynamism. Mol Cell. 2016 Sep 1;63(5):796-810. Epub 2016 Aug 25 PubMed.
- Mateju D, Franzmann TM, Patel A, Kopach A, Boczek EE, Maharana S, Lee HO, Carra S, Hyman AA, Alberti S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017 Jun 14;36(12):1669-1687. Epub 2017 Apr 4 PubMed.
Further Reading
Primary Papers
- Turakhiya A, Meyer SR, Marincola G, Böhm S, Vanselow JT, Schlosser A, Hofmann K, Buchberger A. ZFAND1 Recruits p97 and the 26S Proteasome to Promote the Clearance of Arsenite-Induced Stress Granules. Mol Cell. 2018 Jun 7;70(5):906-919.e7. Epub 2018 May 24 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Boston University School of Medicine
I found this article to be very interesting. We have known that VCP mediates stress granule removal, but the mechanics of this are poorly understand. This manuscript very nicely highlights the role of a protein that appears to function as something like a VCP adapter protein. Importantly, they show that removal of ZFAND1 prevents removal of preformed stress granules (e.g., those formed by TIA1 or FMRP) after arsenite treatment. So the basics of the paper show some nice detailed cell biology. The paper also includes two very interesting extra pieces of information that hint at the complexity of stress granule biology. First, they note that ZFAND1 plays a role in removal of stress granules induced by arsenite, but not heat shock. This very nicely highlights the complexity of RNA granules generally, and stress granules in particular. Proteomics data suggest that stress granules differ by cell type, and this work very nicely highlights physiological differences too—as observed through the lens of ZFAND1-mediated removal. The second pearl of wisdom comes from the analysis of stress granules that remain in the absence of ZFAND1. These stress granules are targeted for removal by autophagy. This observation highlights the different roles played by the proteosome and autolysosomal system in stress granule removal, where the proteasome appears to remove small, dynamic stress granules formed after an acute stress, while the autolysosomal system removes the large, stable stress granules—which I refer to as pathological stress granules (these are what likely accumulate in neurodegenerative diseases).
Make a Comment
To make a comment you must login or register.