As Youth Fades, So Does the Fire of Glycolysis in the Brain
Quick Links
Dogma says that brain metabolism slows down with age: Blood flow, and the total demand for fuel in the form of glucose, decline with passing years. A new study shows that waning glucose uptake is just part of the story—how that glucose gets used also changes dramatically with age.
Writing in the August 1 issue of Cell Metabolism, Manu Goyal, Andrei Vlassenko, and Marcus Raichle of Washington University School of Medicine in St. Louis report that the young brain favors a quick burn, in the form of the partial breakdown of glucose known as aerobic glycolysis. But whole-brain glycolysis declines steadily with normal aging, from its peak at age 20 to nearly zero by age 60. At that age, nearly all of the glucose entering the brain gets metabolized through mitochondria in a longer process that oxidizes glucose completely to water and carbon dioxide. Regional measures indicate that brain areas that start out with the highest rates of glycolysis decline the most, and by age 60, topological differences all but disappear.
“We see that with aging, one of the principal changes in brain metabolism is loss of aerobic glycolysis, and the loss of a youthful pattern of aerobic glycolysis,” said Goyal.
Aerobic glycolysis declines fastest in brain regions that finish developing last. This is consistent with the idea that aerobic glycolysis helps meet the special needs of the maturing brain by supporting synaptic remodeling and myelination, and providing neuroprotection, then lessens once maturation is complete.
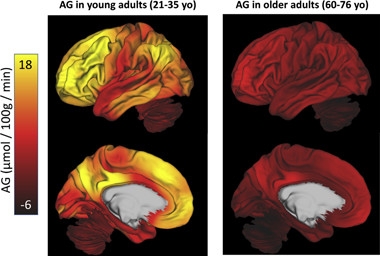
Fading Embers.
Aerobic glycolysis (AG) burns brightly in many areas of the young adult brain (left), but fades to practically nothing in older people (right). [Courtesy of Cell Metabolism, Goyal et al., 2017.]
The highest activity and fastest drops are seen in areas of the default mode network, a set of interconnected brain hubs that are voracious consumers of glucose through life and that experience amyloid deposition and network dysfunction early in Alzheimer’s disease. “This suggests that the cells in these particular parts of the brain are different from others. Maybe hidden within that difference is something that increases their vulnerability over the life span,” said Raichle.
One source of vulnerability may be a diminished buffer against oxidative stress. “While this may be a normal physiological development of old age, an associated aspect of decreasing aerobic glycolysis is a loss of neuroprotection via the pentose phosphate pathway that protects against oxidative stress, thus increasing the risk for oxidative damage. This, in turn, may be a key predisposing factor to neurodegenerative diseases such as Alzheimer’s and Parkinson’s. This may be one reason why these diseases are age-related,” said Richard Caselli, Mayo Clinic Arizona, Scottsdale, in an email to Alzforum. Amyotrophic lateral sclerosis (ALS), too, is marked by oxidative stress in neurons.
Previous work from Raichle’s lab and others demonstrated high levels of aerobic glycolysis in children’s brains, especially in regions undergoing synapse formation and growth. In young adults, glycolysis persists in the resting brain in default mode network regions, which are also the regions most prone to amyloid deposition (Goyal et al., 2014; Sep 2010 news; Research Timeline).
In the new study, Goyal and Vlassenko asked what happens to glycolysis as the brain ages and developmental activity ramps down. The investigators first meta-analyzed previously published quantifications of glucose uptake, oxygen use, and cerebral blood flow in healthy people across age. Some of the 15 studies they drew from used PET scanning; others were classical studies dating back to the 1950s involving the placement of venous and arterial catheters for direct measurement of tracers in blood. The technique, too invasive for general use today, still provides the most quantitative measure of glucose metabolism. Putting the data together, the researchers found an age-related decrease in total glucose use, as expected. However, while the fraction of total glucose burned through the slower route—mitochondrial oxidative phosphorylation—barely changed over the 40-year age span in the study, the fraction going specifically through glycolysis declined steadily from a high of about 20 percent in 20-year-olds, to zero in 60-year-olds.
“The decrease in glucose utilization with age is not new, but the finding that the decrease was largely restricted to loss of aerobic glycolysis was a surprise,” Raichle said.
To explore regional changes, Goyal and colleagues moved to modern PET, using 18F-fluoro-deoxyglucose (FDG) and 15O to measure glucose and oxygen use, respectively, in resting brain scans of 205 healthy men and women between the ages of 20 and 82. The subjects, all cognitively normal, were part of ongoing studies in St. Louis, including some from the Dominantly Inherited Alzheimer Network (DIAN) observational trial, and the ongoing Adult Children Study. Only amyloid-negative subjects were included in the final data analysis of 184 PET sessions in 165 participants.
For each subject, the scientists calculated local rates of glycolysis in 78 brain regions, which revealed that the topology of glycolysis across the brain varied dramatically with age. “Once people got to that 60-and-above age group, you saw some resemblance to the pattern of young adults, but it was just a resemblance,” Goyal said.
The regions that showed highest glycolysis in young adults, such as the precuneus and precentral cortex, fell the most in older people. Areas that started out lower, including the cerebellum, hippocampus, and corpus callosum, remained relatively stable. Because the areas with the highest rates dropped the most, aging effectively “flattened” the topographical map of glycolysis across the brain (see image).
The rapidly declining regions had something in common—they were among the last parts of the brain to mature. That led Goyal to think that the reason the human brain loses aerobic glycolysis across adulthood is because it finally grows up. “It might be that regions with high aerobic glycolysis in young adults are still developing. When they reach maturity, they don’t need aerobic glycolysis anymore, and that’s why we see this flattening,” Goyal said.
In support of this idea, the group had previously demonstrated that, in 16 regions of the brain where they had gene-expression data, the persistence of genes involved in childhood brain development (a characteristic known as neoteny) correlated with glycolysis. In the current work, the age-related decreases also correlated with the degree of neoteny.
The authors suggest that loss of glycolysis could serve as a biomarker for brain aging. But as Marc Dhenain of the CNRS in Fontenay aux Roses, France, pointed out, much of the absolute decrease occurs early, between 20 and 40 years of age. “I think this is more a marker of brain development, and the end of development, rather than aging,” Dhenain said.
Goyal said he thinks of glycolysis as a marker of the brain’s youthfulness. In older people, he sees a lot of variability in the pattern of glycolysis. “In some people in their 70s, the pattern of aerobic glycolysis looks more or less like it does in the young adult, whereas in another older person it looks completely different,” he said. Are those differences important? “My suspicion is that people with a less youthful metabolic brain profile might be more vulnerable to the neurodegeneration seen in Alzheimer’s disease, and might develop it faster,” he said. He and Vlassenko would like to do longitudinal studies to try to answer that question.
In the meantime, they are measuring glycolysis in people with amyloid pathology, both presymptomatic and mildly symptomatic. Vlassenko presented preliminary data at the recent AAIC in London, showing that, in cognitively normal people with amyloid, aerobic glycolysis and tau went in opposite directions. In other words, people with higher glycolysis had less tau pathology.
The current study looked at resting-state metabolism; however, the brain ramps up glycolysis when at work, to fuel synaptic function. Vlassenko said they are starting to look at this dynamic activity as well. A previous study in mice linked neuronal activity, glycolysis, and Aβ deposition (May 2011 news). Goyal is also interested in how changes in glucose use in the default mode network relate to changes in functional connectivity seen on fMRI.
Gwenaëlle Douaud, University of Oxford, England, U.K., said the work provides a strong metabolic hypothesis for what she and others have previously observed: that regions of the brain that develop later are more vulnerable to oxidative stress and age-related neurodegeneration (Douaud et al., 2014). “It will be very interesting to see, as the authors point out, whether this might also be related to loss of myelination. This is something for which Big Data imaging projects, such as the Lifespan Human Connectome Project or the U.K. Biobank Brain Imaging Study, might provide direct, quantifiable answers,” she wrote in an email to Alzforum.
“The findings raise interesting and important questions about the relationship of these physiological changes to other biological changes associated with normal brain aging, such as declines in the density, connectivity, function, or turnover of terminal neuronal fields that innervate these regions, or the density, connectivity, or function of peri-synaptic astroglial cells,” said Eric Reiman, Banner Alzheimer’s Institute, Phoenix. “For instance, prior studies of normal aging have noted a preferential reduction of terminal neuronal fields innervating frontal cortex—not to mention how some of these changes may conspire with other factors in the predisposition to Alzheimer’s disease,” Reiman added.
The study suggests that asessing total glucose uptake with FDG PET offers but a partial view of brain metabolsim. Ai-Ling Lin, University of Kentucky College of Medicine in Lexington, told Alzforum that she believes measuring glycolysis is a better biomarker for aging because it gives more information and changes more dramatically than FDG PET. However, measuring glycolysis with 15O PET is technically complex, and done at only a few centers in the world. Several labs are now developing MRI techniques for measuring oxygen metabolism that will make it easier and more accessible to additional centers, Lin said.
Peter Nelson, a pathologist at the University of Kentucky, Lexington, told Alzforum the study shows why descriptive studies of the human brain remain so important and scientifically valid. “There’s a vogue that is against anything that is not based on clinical hypotheses, and a several-decades-long trend of valuing mouse models. This study shows that you get this incredible benefit from simply studying what goes on in the human brain. Doing it carefully in a large series like this is what it’s all about.”—Pat McCaffrey
References
News Citations
- Brain Aβ Patterns Linked to Brain Energy Metabolism
- Do Overactive Brain Networks Broadcast Alzheimer’s Pathology?
Paper Citations
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014 Jan 7;19(1):49-57. PubMed.
- Douaud G, Groves AR, Tamnes CK, Westlye LT, Duff EP, Engvig A, Walhovd KB, James A, Gass A, Monsch AU, Matthews PM, Fjell AM, Smith SM, Johansen-Berg H. A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci U S A. 2014 Dec 9;111(49):17648-53. Epub 2014 Nov 24 PubMed.
Other Citations
Further Reading
Papers
- Vlassenko AG, Raichle ME. Brain aerobic glycolysis functions and Alzheimer's disease. Clin Transl Imaging. 2015 Feb 1;3(1):27-37. Epub 2014 Dec 10 PubMed.
Primary Papers
- Goyal MS, Vlassenko AG, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TL, Morris JC, Raichle ME. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab. 2017 Aug 1;26(2):353-360.e3. PubMed.
- Dagher A, Misic B. Holding Onto Youth. Cell Metab. 2017 Aug 1;26(2):284-285. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Arizona Alzheimer's Consortium
I am a big fan of the work Marc Raichle and his colleagues have done relating to both the default mode network and aerobic glycolysis. It is an understudied process that may be of great importance to brain development, brain aging, and the predisposition to AD.
In previous studies, Dr. Raichle and his colleagues found that the pattern of aerobic glycolysis in young adults (highest in precuneus, posterior cingulate, and frontal regions) is similar to, and predictive of, the earliest pattern of amyloid plaque deposition at older ages. This raises questions about the mechanisms underlying aerobic glycolysis that conspire with age to provide a foothold for preferential vulnerability to amyloid plaques.
In the present study, they seem to show that those same regions associated with highest aerobic glycolysis in young adults are also associated with the greatest declines with aging, even in the absence of amyloid plaque deposition. The findings raise interesting and important questions about the relationship of these physiological changes to other biological changes associated with normal brain aging, such as declines in the density, connectivity, function, or turnover of terminal neuronal fields that innervate these regions or the density, connectivity, or function of peri-synaptic astroglial cells.
For instance, prior studies of normal aging have noted a preferential reduction of terminal neuronal fields innervating frontal cortex—not to mention how some of these changes may conspire with other factors in the predisposition to Alzheimer’s disease.
While the physiologic phenomenon of aerobic glycolysis (AG) may seem somewhat obscure to a clinical audience, what it represents seems far more tangible. The authors brilliantly articulate the relationship between AG and transcriptional “neoteny,” that is, the relative persistence of childhood developmental gene expression.
At a macro level they describe a gradual “flattening” with age of the topographic variability of AG that characterizes the young brain, perhaps reflecting a reduction in synaptic and dendritic spine development with age.
While this may be a normal physiological development of old age, an associated aspect of decreasing AG is a loss of neuroprotection via the pentose phosphate pathway that protects against oxidative stress, thus increasing the risk for oxidative damage. This, in turn, may be a key predisposing factor to neurodegenerative diseases such as Alzheimer’s and Parkinson’s and may be one reason why these diseases are age-related.
FMRIB Centre, University of Oxford
Aerobic glycolysis (AG) is thought to play a crucial role for the metabolic demands of the developing brain, including those required by synaptic plasticity and myelination, and for neuroprotection. Goyal et al. here demonstrate in this study a clear relationship between brain aging and decrease of AG. These important findings elegantly draw on the previous work from the same team, where they had showed a similar association between AG and brain development.
Notably, in both studies, they have been able to correlate the spatial distribution of AG in the brain to gene expression related to prolonged development. In this current work, they have also shown that age-related, regional changes of AG are very strongly associated with those levels of AG at young adulthood.
Altogether, this provides a strong metabolic hypothesis as to why the regions of the brain that develop later might be the ones more vulnerable to oxidative stress and age-related neurodegeneration (something we, and others, have previously observed).
It will be very interesting to see, as the authors point out, whether this might also be related to loss of myelination. This is something for which Big Data imaging projects, such as the Lifespan Human Connectome Project or the U.K. Biobank Brain Imaging Study, might provide direct, quantifiable answers.
CNRS, CEA, Molecular Imaging Research Center
This article describes the evolution of aerobic glycolysis (AG) in the brain during normal aging. The term aerobic glycolysis refers to the non-oxidative metabolism of glucose before its oxidative phosphorylation (a deeper description of the process can be found in a previous article from the authors, Vlassenko and Raichle, 2015). AG results in the formation of lactate, despite adequate levels of available oxygen that could potentially lead to oxidative phosphorylation processes. PET can provide measurement of not only metabolic rate of glucose (CMRGlu) but also metabolic rate of oxygen (CMRO2), which allows estimation of glucose metabolism outside of oxidative phosphorylation, or aerobic glycolysis (AG). The authors developed methods to measure AG.
In a previous article (Goyal et al., 2014), they showed that AG occurs in the brain in the context of neoteny. This term means that the developmental processes are prolonged after the birth. Cerebral neoteny is one of the particularities of human species. In other words, the developing human brain requires AG. The functions of metabolic products from AG (lactate or other products) are thus probably critical for brain development and plasticity (see for example, Magistretti, 2016).
Here, the authors describe various levels of AG in different brain regions of young subjects and its high occurrence in neotenic cerebral areas. They also show that AG maps flatten with aging, mainly because of AG decrease in the neotenic areas. The age-related decrease of AG occurs independently of the presence of amyloid plaques. However, in previous articles the authors showed that amyloid plaques occurs in the regions with high AG in young subjects (see Vlassenko et al., 2010). They also showed that regions with high AG in young subjects are close to the regions involved in resting state networks which are supposed to be very active in the normal brain (see Vlassenko and Raichle, 2015). These are the same regions in which amyloid deposits in AD patients. The relationship between all these events remains unclear but this article further emphasizes the need to better evaluate the impact of alteration of glucose metabolism and its various sub-entities such as aerobic glycolysis on the occurrence of neurodegenerative processes. The authors have already been working on the relationships between changes in glucose metabolisms and amyloid deposition (see Macauley et al., 2015). In any case, the article is interesting as it points to a mechanism that might be central to the initiation of amyloid deposition in non-genetic forms of AD.
References:
Vlassenko AG, Raichle ME. Brain aerobic glycolysis functions and Alzheimer's disease. Clin Transl Imaging. 2015 Feb 1;3(1):27-37. Epub 2014 Dec 10 PubMed.
Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014 Jan 7;19(1):49-57. PubMed.
Magistretti PJ. Imaging brain aerobic glycolysis as a marker of synaptic plasticity. Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):7015-6. Epub 2016 Jun 17 PubMed.
Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ ) deposition. Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17763-7. PubMed.
Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, Mahan TE, Sutphen CL, Holtzman DM. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest. 2015 Jun;125(6):2463-7. Epub 2015 May 4 PubMed.
Make a Comment
To make a comment you must login or register.