When Perivascular Macrophages Spew SPP1, Microglia Eat Synapses
Quick Links
In neurodegenerative diseases such as Alzheimer’s, aberrantly activated microglia gobble up healthy synapses. What incites these brain immune cells to such mischief? In the February 6 Nature Neuroscience, researchers led by Soyon Hong at the U.K. Dementia Research Institute, University College London, point the finger at a related immune cell—the perivascular macrophage. Normally, these cells monitor the millions of small blood vessels in the brain, tracking clearance of waste materials through the blood-brain barrier. But when exposed to Aβ, PVMs pumped out the proinflammatory cytokine SPP1, the authors found. This triggered nearby microglia to snip synapses. Knocking out SPP1 restrained microglia and protected synapses in both Aβ injection and transgenic mouse models of AD.
- In mice, Aβ prompts perivascular macrophages to express the cytokine SPP1.
- SPP1 stimulates microglia to eat synapses.
- Knocking out SPP1 protects synapses in a mouse model of AD.
Previously, SPP1 was discovered to be a disease-associated microglia (DAM) gene. The cytokine ticks up in the cerebrospinal fluid of people with AD, but its exact role remains undefined. “We identified SPP1 as a regulator of phagocytosis [in AD],” Hong told Alzforum.
Florent Ginhoux at the Agency for Science, Technology and Research, Singapore, called the data exciting. “The crosstalk between microglia and perivascular macrophages through SPP1 in models of early AD is novel, and offers new possible interventions to modulate microglia activity,” he wrote to Alzforum (full comment below).

PVMs Hug Vessels. In mouse hippocampus, a perivascular macrophage (brown) lies along the surface of a brain blood vessel (endothelial cell layer aquamarine, smooth muscle cells purple), inside the basement membrane next to perivascular fibroblasts (green). PVMs are in close contact with astrocytes (gray) and gray matter (pink). [Courtesy of De Schepper et al., Nature Neuroscience.]
Perivascular macrophages have garnered scant attention in AD research. One recent study found that they maintain vessel pulses that wash CSF through the brain, while an earlier one found that in the presence of Aβ, they throttle cerebral blood flow (Nov 2022 news; May 2017 news). Little else is known about their role in AD.

Vascular Villain. In a mouse model of amyloidosis, only perivascular macrophages (green) along blood vessels (gray) in the hippocampus express the phagocytosis-promoting factor SPP1 (red). [Courtesy of De Schepper et al., Nature Neuroscience.]
Hong and colleagues did not set out to study PVMs. First author Sebastiaan De Schepper wanted to investigate the role of secreted phosphorylated protein 1 (SPP1), aka osteopontin, at early stages of disease. He examined APPNL-F mice that were 6 months old, an age when Aβ has begun to accumulate but few plaques have formed. Microglia in these mice’s hippocampi were already devouring synapses, containing sevenfold as much synaptic material as did microglia from wild-type mice. In tandem with this, hippocampal SPP1 was elevated threefold—but not in microglia, as one might expect from a DAM gene. Instead, to De Schepper’s surprise, three-dimensional super-resolution microscopy located the protein to PVMs. Cell-sorting experiments, using cells from mice expressing fluorescent SPP1, confirmed the protein was predominantly in PVMs, with a small amount in perivascular fibroblasts. None turned up in microglia.

The Vessels Have It. SPP1 (red) decorates blood vessels, but is largely absent from parenchyma, in the hippocampi of 6-month-old amyloidosis mice. [Courtesy of De Schepper et al., Nature Neuroscience.]
Notably, SPP1 in PVMs coincided with oligomeric Aβ deposition along the vasculature, as assessed by immunostaining with an oligomer-specific antibody. Curiously, at this 6-month time point, there was almost no oligomeric Aβ in the hippocampal parenchyma.
To explore the relationship between Aβ and SPP1, the authors injected synthetic oligomeric Aβ into the ventricles of wild-type and SPP1 knockout mice. In the wild-type, SPP1 protein in the hippocampus shot up threefold within 18 hours, and microglia swallowed five times as many synapses as did those in uninjected control mice. In SPP1 knockouts, however, microglia left synapses alone after oAβ injection. The authors obtained similar results in transgenic mice. In APPNL-F/SPP1 knockouts, microglia had little appetite for synapses compared to their compatriots in APPNL-F mice.
To Hong and colleagues, the data imply that PVMs switch on SPP1 to promote Aβ clearance, and that the cytokine has unintended, deleterious effects on nearby microglia, causing synapse loss. “SPP1 might be one of the first triggers of disease pathogenesis,” De Schepper said.
Does SPP1 affect microglia directly or indirectly? To gather some clues, the authors compared single-cell RNA-Seq data from PVMs and from microglia isolated from the hippocampi of APPNL-F and APPNL-F/SPP1 knockout mice. Loss of SPP1 suppressed several signaling proteins, including the microglial maturation factor TGFβ1 in PVMs, and its receptors on microglia. “TGFβ1 might be an intermediary molecule influencing communication between cell types,” Hong speculated. The authors are following up with spatial transcriptomic and proteomic studies to gain a clearer view of the cellular crosstalk in the brain environment.
What about humans? As in mice, the authors found SPP1 primarily in PVMs along the vasculature of postmortem AD hippocampus, and less in the parenchyma. This expression pattern is likely due to Aβ, the authors contend, because in neuropathology studies, nearly all AD patients have some vascular amyloid. Extensive vascular deposits are known as cerebral amyloid angiopathy (CAA) and typically co-occur with parenchymal plaques. Intriguingly, severe CAA was recently linked to expression of SPP1, along with a TGFb signaling factor, in blood vessel walls (Grand Moursel et al., 2019).
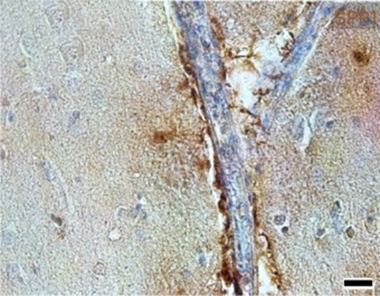
In People, Too. In postmortem AD hippocampus, SPP1 (brown) was mostly found along blood vessels (blue). [Courtesy of De Schepper et al., Nature Neuroscience.]
If vascular amyloid is a trigger for synapse loss, then suppressing SPP1 might help Alzheimer’s patients. This strategy might benefit those with other neurological conditions as well, since SPP1 has been linked to cerebrovascular damage and vascular dementia (Chai et al., 2021).
“One potential therapeutic implication is that targeted reduction of SPP1 in brain may help promote beneficial TREM2-mediated phagocytosis of Aβ by microglia, while minimizing the risk of ‘collateral’ synaptic damage from microglial activation,” wrote Erik Johnson at Emory University, Atlanta. “It will be exciting to see how these findings might translate into new therapeutic approaches for synapse preservation in AD.” In collaboration with Ionis Pharmaceuticals, Carlsbad, California, Hong and colleagues are developing antisense oligonucleotides to test whether knocking down SPP1 can ameliorate disease.—Madolyn Bowman Rogers
References
News Citations
- Perivascular Macrophages: New Target in Aging and Alzheimer’s Disease?
- Do Perivascular Macrophages Mediate Aβ Pathology?
Research Models Citations
Paper Citations
- Grand Moursel L, van der Graaf LM, Bulk M, van Roon-Mom WM, van der Weerd L. Osteopontin and phospho-SMAD2/3 are associated with calcification of vessels in D-CAA, an hereditary cerebral amyloid angiopathy. Brain Pathol. 2019 Mar 13; PubMed.
- Chai YL, Chong JR, Raquib AR, Xu X, Hilal S, Venketasubramanian N, Tan BY, Kumar AP, Sethi G, Chen CP, Lai MK. Plasma osteopontin as a biomarker of Alzheimer's disease and vascular cognitive impairment. Sci Rep. 2021 Feb 17;11(1):4010. PubMed.
Further Reading
News
- Systemic Inflammation: A Driver of Neurodegenerative Disease?
- It’s Not All About You, Neurons. Glia, Blood, Arteries Shine at Symposium
- C1q Shows Promise as Therapeutic Target to Stop Synapse Loss
- Neuronal SRPX2 Spoils Microglial Appetite for Synapses
- With IL-33, Neurons Tempt Microglia to Nibble At Synapses
- Do Microglia Finish Off Stressed Neurons Before Their Time?
- Microglia Control Synapse Number in Multiple Disease States
- Curbing Innate Immunity Boosts Synapses, Cognition
Primary Papers
- De Schepper S, Ge JZ, Crowley G, Ferreira LS, Garceau D, Toomey CE, Sokolova D, Rueda-Carrasco J, Shin SH, Kim JS, Childs T, Lashley T, Burden JJ, Sasner M, Sala Frigerio C, Jung S, Hong S. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer's disease. Nat Neurosci. 2023 Mar;26(3):406-415. Epub 2023 Feb 6 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
This very exciting paper opens new perspectives on how to modulate microglia pathological activity, such as synapse engulfment, that probably contributes to cognitive decline in AD. The identified crosstalk between microglia and perivascular macrophages through SPP1 in early AD models is novel and offers new possible interventions to modulate microglia activity.
It is interesting to see that perivascular macrophages—which are guardians of tissue permeability and, here, sit at the interface between the brain and the circulation—control microglia activity. New questions raised include how to prevent SPP1 expression by such perivascular macrophages, and how to find additional molecular pathways that regulate microglia and perivascular macrophage crosstalk.
University of Edinburgh and UK DRI
This is exciting new study by Soyon Hong‘s group from UK DRI@UCL provides evidence for a key role of border-associated macrophages in modulating microglia function. The paper also demonstrates the power of new binary Cre transgenic mouse models in differentiating the functions of different macrophage populations within the CNS.
It would be exciting to see what role the crosstalk between perivascular macrophages and microglia via osteopontin plays in neuroinflammatory diseases such as multiple sclerosis, where we identified osteopontin-expressing microglia.
Lund University
I found this paper very exciting! In a recent study, we found that higher baseline levels of SPP1 (osteopontin) in cerebrospinal fluid correlated with worse accumulation of tau pathology and cognitive deterioration over time in individuals with preclinical/prodromal AD. This was in contrast to the other studied DAM-associated markers (like sTREM2, AXL, MERTK, GAS6, LPL, CST7, and CSF1), which were instead associated with a more beneficial outcome. I think the current paper by De Schepper et al. helps us understand why SPP-1 has detrimental effects in AD. Further work focusing in the effects of SPP1 on tau pathology in AD might be warranted.
References:
Pereira JB, Janelidze S, Strandberg O, Whelan CD, Zetterberg H, Blennow K, Palmqvist S, Stomrud E, Mattsson-Carlgren N, Hansson O. Microglial activation protects against accumulation of tau aggregates in nondemented individuals with underlying Alzheimer’s disease patholog. Nat Aging 2, 1138–1144 (2022)
Emory University
This is a nice study by De Schepper et al. that highlights the importance of the brain perivascular immune niche in modulating microglial function in the APPNL-F AD mouse model. The authors demonstrate that the immune protein SPP1 is produced predominantly by perivascular macrophages and works as a probable soluble factor to promote microglia phagocytosis of synapses, particularly in response to Aβ oligomers. SPP1 has been shown to be elevated in AD CSF, and PVMs may therefore be one source of this CSF protein.
A number of intriguing questions are raised by the results of this study. What is special about the perivascular space in the hippocampus compared to other brain regions? Might PVM-mediated secretion of SPP1 be one mechanism by which peripheral inflammation or cerebral amyloid angiopathy influence synaptic health? Is PVM-mediated secretion of SPP1 also important in the microglial response to Aβ plaques?
One potential therapeutic implication of these results is that targeted reduction of SPP1 in brain may help to promote beneficial TREM2-mediated phagocytosis of Aβ by microglia while minimizing the risk of “collateral” synaptic damage from microglial activation, particularly given the finding that knockout of SPP1 does not appear to impair microglial phagocytic competence, at least in vitro. It will be exciting to see how these findings might translate into new therapeutic approaches for synapse preservation in AD.
LUMC (Leids Universiteit Medisch Centrum)
This very interesting work suggests a central role of SPP1/osteopontin)in Aβ aggregation along the vasculature.
Interestingly, in patients with Dutch-type cerebral amyloid angiopathy (D-CAA), an early onset hereditary form of CAA due to the APP E693Q mutation, we described an accumulation of osteopontin and TGFβ signaling factor phospho-SMAD2/3 in CAA vessels, as well as the association of col1 protein, a fibrotic protein with vascular amyloid load (Grand Moursel et al., 2019). Upregulation in extracellular matrix pathways in relation to an increase in the Transforming Growth Factor beta (TGFβ) signaling pathway was identified as well in our transcriptomic study in human postmortem D-CAA brain (Grand Moursel et al., 2018).
In D-CAA, accelerated generation or stability of toxic oAβ species has been suggested, although never proven, in patients. Considering De Schepper et al.’s finding that perivascular macrophages act as a major source of osteopontin upon oAβ challenge, toxic oAβ species in D-CAA could be an early trigger of vascular amyloid aggregation resulting in severe CAA. It would be interesting to further investigate if modulation of osteopontin in perivascular macrophages could reduce the CAA phenotype.
References:
Grand Moursel L, van der Graaf LM, Bulk M, van Roon-Mom WM, van der Weerd L. Osteopontin and phospho-SMAD2/3 are associated with calcification of vessels in D-CAA, an hereditary cerebral amyloid angiopathy. Brain Pathol. 2019 Mar 13; PubMed.
Grand Moursel L, van Roon-Mom WM, Kiełbasa SM, Mei H, Buermans HP, van der Graaf LM, Hettne KM, de Meijer EJ, van Duinen SG, Laros JF, van Buchem MA, 't Hoen PA, van der Maarel SM, van der Weerd L. Brain Transcriptomic Analysis of Hereditary Cerebral Hemorrhage With Amyloidosis-Dutch Type. Front Aging Neurosci. 2018;10:102. Epub 2018 Apr 13 PubMed.
Make a Comment
To make a comment you must login or register.