When Neurons Get Excited, They Spill α-Synuclein
Quick Links
Could synaptic release help α-synuclein sow its seeds of destruction? That’s one possible upshot from a study by Kaoru Yamada and Takeshi Iwatsubo of the University of Tokyo, who report that neuronal activity boosts the secretion of α-synuclein in the mouse brain. Using in vivo microdialysis to track the protein in brain interstitial fluid (ISF), the researchers discovered that increasing excitatory synaptic activity caused the concentration of extracellular α-synuclein to jump, while dampening activity reduced it. The protein exited cells as a high-molecular-weight species of uncertain makeup. Published in the February 22 Molecular Neurodegeneration, the results suggest a physiological route to extracellular accumulation of α-synuclein, itself a possible contributor to toxicity, or cell-to-cell spread of pathology in Parkinson’s disease and other synucleinopathies.
- Neuronal activity regulates extracellular α-synuclein in brain.
- Released α-synuclein appears to be multimeric, or complex with other proteins.
- Disturbance of physiological release could figure in spread of α-synuclein.
The results are no big surprise, given that α-synuclein is enriched in synaptic regions of neurons and has been implicated in synaptic vesicle function, said Patrik Brundin of the Van Andel Research Institute in Grand Rapids, Michigan. However, Brundin told Alzforum, “It is a bit unexpected that a significant portion of the released protein seems to be present as either a multimer of synuclein, or associated with another protein. If it is oligomerizing, that is surprising.”
Even in healthy brains, neurons secrete several potentially pathological proteins into the ISF, presumably in harmless or physiologically useful forms. Tau, for one, ramps up its release when neurons fire, as Yamada was first to discover while a postdoc in David Holtzman’s lab at Wash U (Feb 2014 news). She knew that α-synuclein is also released in the brain (Sep 2011 news), and wondered if it might exhibit the same behavior.
To test that idea, Yamada first studied α-synuclein release from cultured mouse cortical neurons, using a commercial ELISA to detect α-synuclein in the media. When she inhibited the cells’ action potentials with tetrodotoxin (TTX), α-synuclein secretion dropped by about 30 percent, whereas stimulating excitatory transmission with glutamate increased extracellular α-synuclein by 40 percent. Adding glutamate receptor blockers to the cultures both decreased basal and evoked α-synuclein secretion. Yamada detected no signs of toxicity in any of these experiments, and neuronal levels of α-synuclein remained steady.
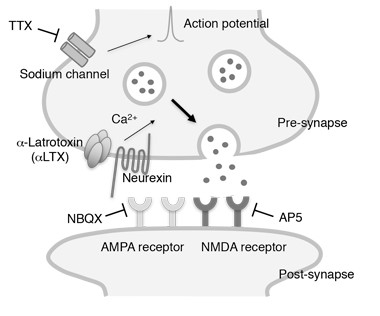
Yamada next looked in the brain. She used microdialysis to track changes in mouse hippocampus α-synuclein levels in response to pharmacological agents. Infusing picrotoxin, a GABA A receptor antagonist that causes excitatory neural activation, increased ISF α-synuclein, which peaked at four times basal levels after six to nine hours of treatment. In contrast, infusing TTX to inhibit neuronal firing reduced ISF α-synuclein by about 70 percent within 12 hours of treatment. The results suggest that ISF α-synuclein originates mainly from activity-dependent secretion, and turns over rapidly. By comparison, secreted tau lingers for weeks, with a half-life of 11 days.
How might neuronal activity promote α-synuclein secretion? One way could be through release from synaptic vesicles. In support of this idea, cultured neurons released more α-synuclein in response to treatment with α-latrotoxin, which promotes calcium-dependent vesicle fusion without depolarization. While suggestive, this experiment does not prove activity-dependent release occurs via synaptic vesicles. Other mechanisms, including release in exosomes, may also play a part, the authors write (Emmanouilidou et al., 2010; Emmanouilidou et al., 2016).
What form of α-synuclein do neurons secrete? Controversy persists about the protein’s makeup in cells—some believe the 14 kDa protein exists mainly as cytoplasmic monomers or membrane-bound multimers, while others detect tetrameric assemblies (Oct 2016 news). In this study, the secreted protein appeared bigger than a monomer. Microdialysis with a large (1,000 kDa) cutoff membrane retrieved ample α-synuclein from the ISF, but when Yamada switched to a 30 kDa cutoff membrane, she collected none. Size exclusion chromatography of the ISF revealed a sharp peak of α-synuclein reactivity around 60 kDa, about the same size as the tetramer identified in the labs of Dennis Selkoe of Harvard University, and others (Oct 2016 news; Aug 2011 news).
If the secreted form were a tetramer, that would be an important discovery, Selkoe wrote to Alzforum. “The existence of a potentially metastable tetramer in normal ISF is intriguing although not proven definitively here,” he wrote (see full comment below).
“We don't know what high-molecular-weight synuclein represents,” wrote Yamada in an email to Alzforum. “The molecular weight matches the reported tetramer, but other interpretations, such as lipid-bound synuclein, are also possible.”
Could this pathway contribute to the transsynaptic spread of α-synuclein pathology? The authors speculate that elevation of the protein’s concentration at synapses might ultimately facilitate oligomer formation and the propagation of pathology, as proposed for tau (Jun 2016 news). Before making that leap, Brundin said, it will be critical to do a careful analysis of the exact nature of the secreted α-synuclein to determine what the ISF species are, and if they are capable of forming oligomers or fibrils.—Pat McCaffrey
References
News Citations
- Neurons Release Tau in Response to Excitation
- Brain Microdialysis Reveals Tau, Synuclein Outside of Cells
- Fatty Acid Greases the Wheels for α-Synuclein Multimers
- An α-Synuclein Twist—Native Protein a Helical Tetramer
- Excited Neurons Release More Aberrant Tau
Paper Citations
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010 May 19;30(20):6838-51. PubMed.
- Emmanouilidou E, Minakaki G, Keramioti MV, Xylaki M, Balafas E, Chrysanthou-Piterou M, Kloukina I, Vekrellis K. GABA transmission via ATP-dependent K+ channels regulates α-synuclein secretion in mouse striatum. Brain. 2016 Mar;139(Pt 3):871-90. Epub 2016 Feb 8 PubMed.
Further Reading
Primary Papers
- Yamada K, Iwatsubo T. Extracellular α-synuclein levels are regulated by neuronal activity. Mol Neurodegener. 2018 Feb 22;13(1):9. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Co-Director, Brigham and Women's Hospital's Ann Romney Center for Neurologic Diseases
The authors compared a 30 kDa to a 1,000 kDa MW cutoff dialysis membrane during brain microdialysis (Fig. 2 l-o), and suggest that the form of α-synuclein they found in ISF was >30 kDa, i.e., good passage of recombinant monomers (14 kDa) but not this natural α-synuclein species. They then sized the latter by non-denaturing SEC, suggesting a rather sharp peak at ~60-70 kDa.
This could be consistent with a physiological tetramer such as we and others have described in neurons and erythrocytes in the last few years (e.g., Bartels et al., 2011; Dettmer et al., 2015), though the authors do not link their findings to this earlier work.
However, there are two caveats: a) SEC is known not to distinguish α-synuclein monomers from tetramers well, due to the large hydrodynamic radius of the monomer, which leads to its elution at ~60 kDa on SEC columns; and b) the normal tetramers we have described are detectable intraneuronally by intact-cell cross-linking and apparently require “molecular crowding” for stability, such that cell lysis or brain homogenization largely depolymerizes the tetramer in dilute solution (Dettmer et al., 2013).
We speculate that a small lipid moiety binds to the α-synuclein tetramer intracellularly to help stabilize it, and this “limiting factor” is diluted/lost upon cell lysis (see also Kim et al., 2018). Nevertheless, the species the authors describe in ISF is presumably a normal form of α-synuclein (the mice are wild-type and healthy), so it is possible that a lipid moiety stays bound to the tetramer during physiological secretion by neurons, which would be an important discovery. So, the existence of a potentially metastable tetramer in normal ISF is intriguing although not proven definitively here. Cross-linking a sample of ISF and then running it on denaturing SDS-PAGE could reveal whether the ISF species then runs at 60 kDa and can thus be assumed to be a tetramer in vivo.
Parenthetically, the authors emphasize the role of abnormal extracellular α-synuclein species being physically transported from neuron to neuron during PD, but a) their species is normal, and b) this hypothesis for PD is not consistent with all available evidence (Walsh and Selkoe, 2016).
References:
Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011 Aug 14;477(7362):107-10. PubMed.
Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, Sanderson JB, Jaenisch R, Bartels T, Selkoe D. Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015 Jun 16;6:7314. PubMed.
Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D. In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells. J Biol Chem. 2013 Mar 1;288(9):6371-85. Epub 2013 Jan 14 PubMed.
Kim S, Yun SP, Lee S, Umanah GE, Bandaru VV, Yin X, Rhee P, Karuppagounder SS, Kwon SH, Lee H, Mao X, Kim D, Pandey A, Lee G, Dawson VL, Dawson TM, Ko HS. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc Natl Acad Sci U S A. 2018 Jan 23;115(4):798-803. Epub 2018 Jan 8 PubMed.
Walsh DM, Selkoe DJ. A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat Rev Neurosci. 2016 Apr;17(4):251-60. PubMed.
Washington University
The Yamada paper clearly demonstrates that neuronal activity regulates release of α-synuclein. They use a similar microdialysis system that we have used to show that activity regulates Aβ, and that Dr. Yamada has used to show activity regulates tau.
Activity seems to have a larger effect on α-synuclein release than it does for either Aβ or tau. The latrotoxin experiments are particularly interesting, demonstrating that synaptic vesicle exocytosis alone is sufficient to increase α-synuclein levels.
Of course the missing component here is HOW activity causes α-synuclein release. It is a synaptic vesicle-associated protein, but on the cytoplasmic side of the membrane, so there is no reason it would have to get secreted at all. Figuring out how it gets out of the cell will be very important. And of course what species of α-synuclein is secreted, monomer or aggregates, which has implications for synuclein propogation between cells.
Make a Comment
To make a comment you must login or register.