Turning Peripheral Stem Cells into Amyloid-Gobbling Brain Phagocytes
Quick Links
For brain microglia struggling to keep amyloid plaques under control, help could be on the way. In a study published this week in PNAS, Richard Lerner and colleagues at The Scripps Research Institute in La Jolla, California, identify an antibody that triggers mouse bone marrow myeloid progenitors to become phagocytic microglia-like cells, which then make their way to the brain. In a mouse model of AD, the cells clustered around amyloid deposits and reduced plaque load.
- Antibody induces bone-marrow cells to assume a microglia-like phenotype.
- In vitro, the cells phagocytose Aβ.
- In AD mice, cells home to the brain and reduce plaque load.
Scientists in the field called the approach new and interesting, and the results promising, but agreed much work remains to be done to evaluate whether a stem cell-based approach to amyloid clearance could work in people.
“Any type of treatment that will induce recruitment of peripheral myeloid cells toward plaques and remove them is a big deal,” said Oleg Butovsky of Brigham and Women’s Hospital, Boston, who was not involved in the work.
The findings grew out of a problem Lerner saw with stem-cell therapies—it’s not enough to generate the desired type of cell; the cells also must be directed to where they are needed. “After embryogenesis, the ‘go there’ part is shut down,” Lerner told Alzforum. He said controlling migration is the other half of stem-cell therapy.
To address that, Lerner devised a screen for antibodies that induce stem cells to not only differentiate but also migrate to different tissues. After expressing antibodies on bone-marrow stem cells, he put those cells in mice and looked for the ones that gained the ability to migrate to the brain or other tissues. “In a normal selection, you look at a cell population and find something that’s different,” he told Alzforum. “In this migration-based selection, the cells self-purify because they run away from the bone marrow, and take up residence in other tissues.”
First author Kyung Ho Han started with a lentivirus expression library comprising 100 million different single-chain antibody genes. The antibodies, derived from human cells, were engineered to contain a membrane-spanning region so that they would be expressed on the cell surface (Xie et al., 2013). Han infected freshly isolated mouse bone-marrow cells with the library, then transplanted the entire batch of infected cells into mice whose own bone marrow had been destroyed by whole-body irradiation. After a week, he used PCR to detect traces of the antibody genes in different tissues. Of 60 different genes detected, Han found one, dubbed B1, six times in brain tissue from different mice, and never in spleen, liver, or heart.
To see if the antibody encoded by B1 induced migration of infected bone marrow cells to the brain, the investigators transduced B1 into bone-marrow cells marked with the red fluorescent protein mCherry. One week after those cells were put into irradiated mice, the investigators found abundant red cells in brain, over and above the few seen in control animals transplanted with uninfected mCherry bone marrow. The mCherry cells concentrated in the substantia nigra, the hippocampus, and the hypothalamus.
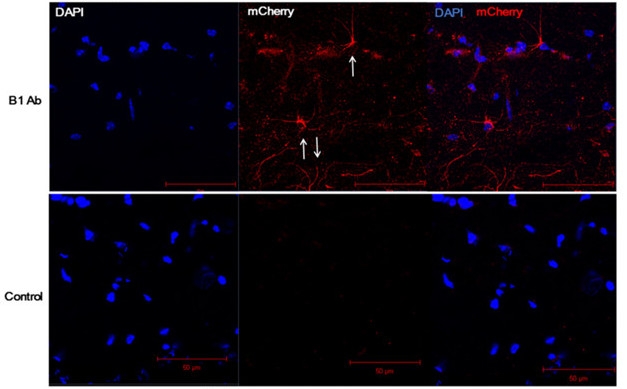
Code Red. mCherry (red) bone marrow cells transduced with B1 antibody (top panel) migrate to the brain in irradiated mice (arrows), while non-transduced cells do not (bottom panel). [Courtesy of Han et al., PNAS.]
Morphologically, the mCherry cells in the brain looked like microglia, and some bound an antibody to the proposed microglial marker TMEM119 (Satoh et al., 2016; Bennett et al., 2016). To study the cells in more detail, Han and colleagues expressed and purified the B1 antibody, and used it to differentiate mouse bone-marrow cells or human CD34+ monocyte precursors in vitro. RNA-Seq profiling revealed an expression profile that resembled human microglia and was distinct from cells treated with macrophage colony-stimulating factor, a cytokine that induces macrophage differentiation. The induced human cells showed a pro-inflammatory phenotype and were phagocytic, avidly gobbling up latex beads and Aβ42 peptides in vitro.

Branching Out.
B1 treatment of human CD34+ progenitor cells produced ramified, microglia-like cells that could phagocytose Aβ. [Courtesy of Han et al., PNAS.]
To ask if the microglia-like cells attack amyloid in vivo, the researchers repeated their transplantation experiments using APP/PS1 mice. They infused B1-transduced bone marrow cells into irradiated eight-week-old animals, and then waited. After six months, the B1 mice harbored approximately 60 percent less amyloid in their brains than animals transplanted with control bone marrow. The animals also had more microglia and fewer astrocytes than irradiated controls, as indicated by Iba1 and GFAP reactivity.
In non-irradiated mice, or in mice irradiated while wearing a lead helmet to protect the integrity of the blood-brain barrier, circulating monocytes do not readily penetrate the brain (Nov 2007 news). Consistent with this, Lerner’s group found that B1 cells were unable to enter the brains of wild-type mice irradiated while wearing the helmet. But what would happen in diseased mice, whose blood-brain barriers might be stressed? In a crucial experiment, Han found that B1-induced cells accessed the brain even in non-irradiated eight-month-old APP/PS1 mice. The investigators did not determine whether plaque was reduced; Lerner said they are doing those experiments now. He will also test if the B1 cells alter the mice’s behavior, he told Alzforum.
Does B1 treatment induce bona fide microglia and if so, what type? This is difficult to evaluate based on the information in the paper, Ido Amit, Weizmann Institute of Science, Israel, wrote to Alzforum. “The gene expression comparisons are not quantitative, and difficult to assess given the current data,” he wrote. “The true comparison that I would use here is RNA-Seq analysis of the B1 antibody population compared to in vivo extracted microglia—single-cell analysis would be even better.”
Butovsky brushed this question aside. “I don’t think these cells are the real deal, but I don’t care. If, as claimed in the study, this antibody induces a type of cell that can migrate to plaque in non-irradiated mice, that’s important. If this is associated with plaque removal, that’s huge. Whether the cells become microglia is not as important.”
Lerner’s group identified the target of the B1 antibody as the cytoskeletal protein vimentin, which they detected on the surface of CD34+ cells. Bone marrow from vimentin-knockout mice did not produce microglia-like cells in response to B1, providing further evidence of a role for the protein in the differentiation and migration processes. Interestingly, a commercial vimentin antibody could not induce microglia in bone marrow, suggesting that B1 possesses additional stimulatory capabilities.
Terrence Town, University of Southern California in Los Angeles, was intrigued by the results. His own work established that inhibiting TGFβ signaling in mice allows peripheral macrophages to enter the brain and clear plaque (Jun 2008 news). “The authors here are getting to the same destination via a different road,” Town told Alzforum. He was impressed that the cells targeted plaque in older, non-irradiated APP/PS1 mice, but stressed the need to know if they caused amyloid clearance.
Todd Golde, University of Florida, Gainesville, found the screening method interesting, but said the results raise many questions. Golde was not convinced by the data on amyloid reduction, because of a lack of detail about the experiments, including information on the numbers and sexes of animals used. "This is a hypothesis-generating paper,” he said, adding, “The antibody will be a very useful tool if the results can be replicated.”
The work may stimulate new interest in stem-cell approaches to AD. Researchers are sharpening their skills at producing microglia-like cells from induced pluripotent stem cells (Jul 2016 conference news), and more recently, from monocytes (Jan 2018 news). Lerner sees similarities between the B1 work and new cancer therapies that use modified chimeric antigen receptor (CAR)-T cells. “I like to think we could take peoples’ bone marrow, transform the cells into microglia, and put them back, where they would go to the brain and clear up amyloid. Two years ago you would say we cannot do this, but now a similar thing has been done in thousands of people with cancer.” (For a review of CAR-T see Ramello et al., 2017.)
Would this be better than current efforts to clear plaques with anti-Aβ antibodies? “The Aβ antibody trials have been iffy, and before I spent another billion on those trials, I might think about doing something like this,” Lerner said. “Maybe we can take a lesson from the immunologists who have been after cancer with antibodies for 50 years, and now find it looks like the cells will do a better job.”—Pat McCaffrey
References
Research Models Citations
News Citations
- The Brain and Microglial Recruitment—Think Local, Not Global
- Macrophages Storm Blood-brain Barrier, Clear Plaques—or Do They?
- Induced Microglia Make Debut at Keystone Symposium
- New Microglial Model May Aid Genetic Studies, Drug Screening
Paper Citations
- Xie J, Zhang H, Yea K, Lerner RA. Autocrine signaling based selection of combinatorial antibodies that transdifferentiate human stem cells. Proc Natl Acad Sci U S A. 2013 May 14;110(20):8099-104. Epub 2013 Apr 23 PubMed.
- Satoh J, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T, Saito Y. TMEM119 marks a subset of microglia in the human brain. Neuropathology. 2016 Feb;36(1):39-49. Epub 2015 Aug 6 PubMed.
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):E1738-46. Epub 2016 Feb 16 PubMed.
- Ramello MC, Haura EB, Abate-Daga D. CAR-T cells and combination therapies: What's next in the immunotherapy revolution?. Pharmacol Res. 2017 Dec 1; PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Han KH, Arlian BM, Macauley MS, Paulson JC, Lerner RA. Migration-based selections of antibodies that convert bone marrow into trafficking microglia-like cells that reduce brain amyloid β. Proc Natl Acad Sci U S A. 2018 Jan 16;115(3):E372-E381. Epub 2018 Jan 2 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.