Stress Granules: No Incubator for Inclusions, After All?
Quick Links
TDP-43 proteinopathy rears its ugly head in most patients with amyotrophic lateral sclerosis and nearly half of those with frontotemporal dementia, not to mention many cases of Alzheimer’s disease and traumatic brain injury. Still, it’s been hard to recapitulate the dynamics of toxic TDP-43 inclusions in cell or animal models. A paper published February 27 in Neuron offers a new approach. Attaching a photosensitive protein that oligomerizes under blue light, researchers led by Christopher Donnelly, University of Pittsburgh, coaxed TDP-43 to condense at will. The chimera condensed into liquid droplets, where it formed solid inclusions that were toxic to cells. However, when it bound RNA, TDP-43 slid into a different type of liquid droplet, namely stress granules, where it remained soluble. Adding RNA to cultured human neurons from induced pluripotent stem cells prevented TDP-43 aggregates from forming. The results may shift how researchers think about TDP-43 aggregation.
- New optogenetic model lets researchers control TDP-43 aggregation.
- TDP-43 inside stress granules stays soluble, but TDP-43 outside becomes insoluble.
- Treating cells with small RNAs keeps inclusions from forming.
“For years, the field thought persistent stress granules could be the bad guys, seeding pathology,” said Steven Boeynaems, Stanford University, who was not involved in the study. “This paper provides evidence for a more nuanced role of stress granules in ALS pathology, suggesting nucleation of pathological aggregates may be happening outside of them.”
Clifford Brangwynne, Princeton University, New Jersey, agreed. “This paper shows some evidence that the TDP-43 in stress granules remains dynamic, and does not seem to have features of irreversible aggregates, whereas TDP-43 inclusions outside of SGs may be causing the real problems. This is a solid paper and very well done.”
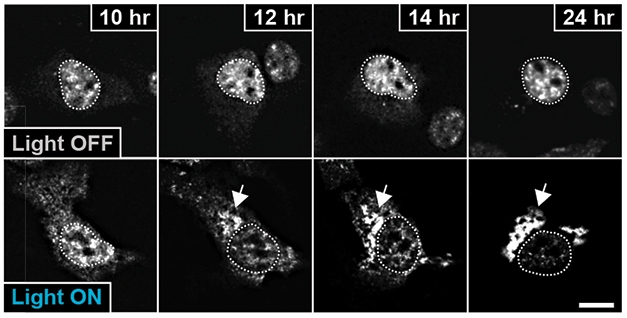
Lights, Microscope, Action. In 24 hours of darkness (top), light-sensitive optoTDP-43 (bright spots) stays inside the nucleus (dotted circle). Exposed to blue light (bottom), optoTDP-43 leaves the nucleus and aggregates in the cytoplasm. [Courtesy of Mann et al., 2019. Neuron.]
Two years ago, Brangwynne introduced a new way to convene RNA-binding proteins (Jan 2017 news). A photoreceptor called cryptochrome 2 (Cry2), found in the plant Arabidopsis thaliana, oligomerizes when exposed to blue light. When hitched to low-complexity domains of the ALS proteins FUS or HNRNPA1, Cry2 brought these motifs together, causing them to phase-separate into droplets, a process known as liquid-liquid phase separation (LLPS; Mar 2017 news). With the flick of a switch, Brangwynne and colleagues could form and dissociate assemblies of RNA-binding proteins, or portions of them, at will.
Death by Light. In dark conditions, optoTDP-43 stays in the nuclei of cultured neurons (top) and neuronal processes look normal as judged by fluorescent reporter driven by a synapsin promoter (purple). Upon exposure to blue light, optoTDP-43 aggregates outside the nucleus, the neuron exhibits progressive blebbing, loss of synuclein, and ultimately bursts.
In the current paper, Donnelly and colleagues extended this model by using a modified version of Cry2 called Cry2olig that aggregates faster and more robustly (Taslimi et al., 2014). They attached it to the N-terminal end of TDP-43 and affixed a red fluorescent tag to the LCD. With this model, they could control when and where TDP-43 inclusions formed and study what makes the protein aggregate.
To start, first author Jacob Mann expressed this fusion protein, called optoTDP-43, in HEK293 cells and watched them under a fluorescent microscope. OptoTDP-43 mostly kept to nuclei and stayed soluble while cells remained in darkness; it drained out of the nucleus and clumped up in the cytoplasm in cells exposed to blue light for 24 hours (see image above). These inclusions matched the characteristics of those seen in patient tissue: They were insoluble to detergent, contained the protein p62, co-localized with ubiquitin, and the TDP-43 became hyperphosphorylated. The inclusions also roped in endogenous TPD-43.
To examine which domain was responsible for forming these inclusions, Mann did a type of reverse engineering, attaching the light-responsive Cry2 to various portions of TDP-43. Only when fused to the LCD region did chimeras form droplets. This fits with Brangwynne’s data showing the LCDs of other RNA-binding proteins causes the proteins to phase-separate. If the TDP-43 LCD contained any of three ALS mutations—M337V, Q331K, or A321V—inclusions formed faster.
The same light show failed to phase-separate full-length optoTDP-43 in cells, however. Given that regular TDP-43 easily forms liquid droplets in cell-free media, the authors wondered which component in the cell might prevent phase separation (Conicella et al., 2016). Could it be RNA? To find out, they tacked RNA-recognition motifs and LCDs onto Cry2Olig and expressed the chimeras in HEK293 cells. Blue light failed to induce phase transitions; however, if the RNA-binding domains were mutated to be non-functional, then the fusion proteins clustered together right away. The data suggest that RNA binding blocked phase transitions of the LCDs.
If that is true, could adding RNA prevent light-induced inclusions from forming? When Mann treated HEK293 cells with total HEK293 RNA and flashed them with light, inclusion formation dropped 28 percent relative to untreated cells. In a test tube, the liquid droplets formed by purified TDP-43 went away as Mann added increasing amounts of RNA. If the TDP-43 contained mutations that disable RNA binding, liquid droplets formed regardless of how much RNA was added.
How would modulating RNA binding affect recruitment of TDP-43 to stress granules? Mann expressed wild-type TDP-43, or TDP-43 with a mutated RNA binding domain, in HEK293 cells, then coaxed stress granules to form using a heat shock. Normal TDP-43 wound its way into stress granules and stayed fluid. However, TDP-43 that couldn’t bind RNA accumulated separately in the cytoplasm. A fraction of wild-type protein that didn’t bind RNA aggregated in inclusions that lacked stress granule markers as well. In these bodies, TDP-43 became hyperphosphorylated and co-localized with p62. The results suggest that as long as TDP-43 binds RNA and gets recruited to stress granules, it stays soluble, but unattached TDP-43 accumulates in insoluble aggregates distinct from stress granules (see image below).
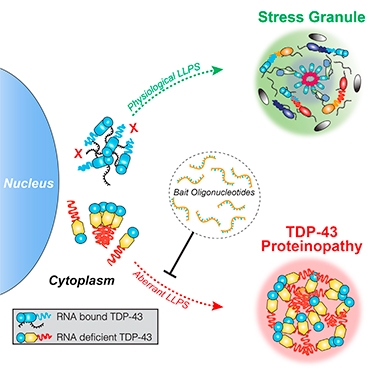
Safer When Hitched. Bound to RNA, cytoplasmic TDP-43 makes its way to stress granules (top). Unattached protein forms aggregates in distinct liquid droplets (bottom). Oligonucleotides that target TDP-43 can prevent inclusions from forming. [Courtesy of Mann et al., 2019. Neuron.]
The light-induced TDP-43 inclusions appeared to be toxic to cells. When the authors differentiated optoTDP-43-expressing human neural progenitor cells into cortical neurons, 90 hours of light stimulation caused them to form fluorescent inclusions in the cytoplasm and 4.3-fold more neurons died after the light treatment than after being kept dark (see movie above). However, if the authors pretreated those cells with increasing doses of a small ribonucleotide designed to fit the RNA-binding pocket of TDP-43, then light treatment induced fewer optoTDP-43 assemblies. In cultured cortical neurons, the ribonucleotide reduced the number of optoTDP-43 clumps that formed and reduced TDP-43 neurotoxicity.
Altogether, the data suggest that TDP-43 forms aggregates outside of stress granules, and only when it’s not bound to RNA. They also imply that an RNA-oligonucleotide treatment could prevent these inclusions from forming.
“We think this could point the way to a novel oligonucleotide-based therapy,” Donnelly told Alzforum. “We could use a specific sequence for which TDP-43 has high affinity, soak up mislocalized TDP-43 in the cytoplasm, and prevent it from forming inclusions.” Boeynaems agreed. “Maybe stress granules shouldn’t be a direct therapeutic target, but it’s these out-of-stress-granule nucleation events that we should try to target,” he said. Brangwynne thinks that this mechanism of aggregating outside of stress granules could generalize to other RNA-binding proteins as well.
“It’s a really interesting strategy,” agreed Robert Baloh, Cedars-Sinai Medical Center, Los Angeles. While it would presumably be a bad idea to block TDP-43 function entirely, Baloh thinks modulating it with oligonucleotides that suppress toxic aggregation while leaving normal activities alone is an innovative approach. Overall, he praised the model, even if it’s artificial. “If they hadn’t hit all the bases by demonstrating that their light-induced system generated TDP-43 aggregates with numerous properties expected from toxic aggregation, people might have been more skeptical,” he told Alzforum. “But I think most will agree they are really onto something.” Whether these dynamics play out in mouse models and in people still remains to be determined, he added.
The results dovetail with previous work suggesting stress granules are initially protective (McGurk et al., 2018). However, over the long term, persistent stress granules may push TDP-43 toward the pathological state (Zhang et al., 2018).
“The timeline of events seems crucial,” Boeynaems told Alzforum. “Stress granules are initially a good response, but it may be hard to keep proteins soluble if they remain in LLPS droplets long-term.” —Gwyneth Dickey Zakaib
References
News Citations
- Forming FUS Droplets with a Flash of Light
- ALS Dipeptides Drive Liquid-Liquid Phase Separation, Stress Granule Formation
Paper Citations
- Taslimi A, Vrana JD, Chen D, Borinskaya S, Mayer BJ, Kennedy MJ, Tucker CL. An optimized optogenetic clustering tool for probing protein interaction and function. Nat Commun. 2014 Sep 18;5:4925. PubMed.
- Conicella AE, Zerze GH, Mittal J, Fawzi NL. ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure. 2016 Sep 6;24(9):1537-49. Epub 2016 Aug 18 PubMed.
- McGurk L, Gomes E, Guo L, Mojsilovic-Petrovic J, Tran V, Kalb RG, Shorter J, Bonini NM. Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Mol Cell. 2018 Sep 6;71(5):703-717.e9. Epub 2018 Aug 9 PubMed.
- Zhang P, Fan B, Yang P, Temirov J, Messing J, Kim JH, Taylor JP. OptoGranules reveal the evolution of stress granules to ALS-FTD pathology. bioRχiv. June 18, 2018.
Further Reading
Papers
- Harrison AF, Shorter J. RNA-binding proteins with prion-like domains in health and disease. Biochem J. 2017 Apr 7;474(8):1417-1438. PubMed.
- Boeynaems S, Gitler AD. Pour Some Sugar on TDP(-43). Mol Cell. 2018 Sep 6;71(5):649-651. PubMed.
Primary Papers
- Mann JR, Gleixner AM, Mauna JC, Gomes E, DeChellis-Marks MR, Needham PG, Copley KE, Hurtle B, Portz B, Pyles NJ, Guo L, Calder CB, Wills ZP, Pandey UB, Kofler JK, Brodsky JL, Thathiah A, Shorter J, Donnelly CJ. RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43. Neuron. 2019 Apr 17;102(2):321-338.e8. Epub 2019 Feb 27 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.