Sigh of Relief? Lung Effects of LRRK2 Inhibitors are Mild.
Quick Links
Proponents of LRRK2 inhibitor programs may breathe easier following a paper in the April 22 Science Translational Medicine. A collaboration led by researchers at the Michael J. Fox Foundation found that three different inhibitors of this Parkinson’s-linked kinase caused morphological changes in the lungs of macaque monkeys but did not compromise their respiratory function, at least over a period of two weeks. No morphological changes were detected in the kidney or brain.
- LRRK2 inhibitors cause lung cells to swell.
- This does not affect breathing, and goes away when treatment stops.
- Clinical trials will need to monitor pulmonary function.
“Overall, this is reassuring,” said Mark Cookson, National Institute on Aging, Bethesda, Maryland. He was not involved in the work. “This tells us we need to monitor target populations with appropriate lung-safety testing, but this should not disqualify these drugs from clinical trials,” he told Alzforum.
Leucine-rich repeat kinase 2 has basked in the limelight since 2004, when scientists discovered that variants in the LRRK2 gene cause familial Parkinson’s (Oct 2004 news). Later, LRRK2 variants were found to increase the risk for developing idiopathic forms of the disease as well (Gilks et al., 2005). How its protein predisposes to PD has been debated because it is massive, sporting many different functional domains. In people, LRRK2 is widely expressed but highest in the kidney, lung, and lymph (Biskup et al., 2007).
Over time, research has converged on kinase function being most germane for PD. The most common risk variant, G2019S, lies in the catalytic site, where it increases kinase activity. So do variants in other domains of the protein that have been linked to PD (Nov 2005 news; Sheng et al., 2012). If a boost in kinase activity increases the chances of getting PD, then, researchers predicted, blocking this activity should prevent or slow it. The hunt for such drug candidates was on.
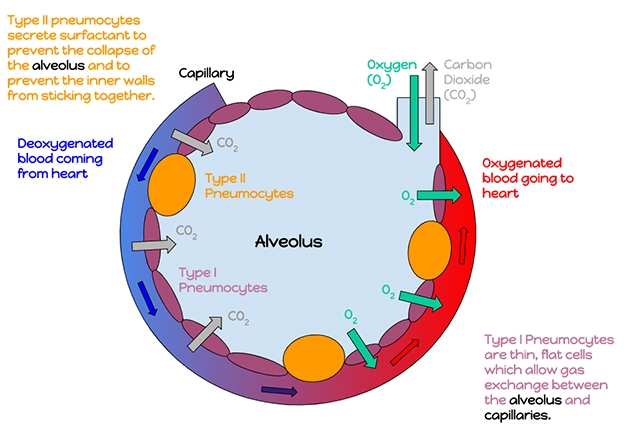
LRRK2 and Pneumocytes. In the alveolus, type II pneumocytes secrete surfactants that facilitate the diffusion of oxygen into the blood. When LRRK2 is blocked, they develop cytoplasmic vacuoles. [Courtesy of Katherinebutler1331 - Own work, CC BY-SA 4.0.]
Scientists felt a bit deflated when they realized that knocking out LRRK2 in rodents caused morphological changes in the lungs, kidneys, and spleen (Ness et al., 2013; Tong et al., 2012; Baptista et al., 2013). Would inhibiting the kinase do the same? Sure enough, GNE-7915 and GNE-0877, LRRK2 inhibitors developed at Genentech, South San Francisco, caused lysosomal-like organelles called lamellar bodies to accumulate in type II pneumocytes of cynomolgus monkeys (Fuji et al., 2015).
Type II pneumocytes are the most numerous of the three cell types found in lung alveoli, the other two being type I pneumocytes and macrophages. Type II pneumocytes secrete a surfactant that reduces surface tension, stops the alveolar lining from sticking to itself, and improves diffusion of oxygen and carbon dioxide across type I pneumocytes (see image above). A mixture of phospholipids, the surfactant extrudes from the same lamellar bodies that increased in size and number in response to the LRKK2 inhibition or in LRRK2 knockouts. Researchers led by Ryan Watts, who then worked at Genentech, now at Denali, concluded that pulmonary toxicity may be a critical safety liability for LRRK2 kinase inhibitors.
Still, whether this morphological change compromises lung function was unclear. Marco Baptista at MJFF wanted to find out. Baptista also wanted to know if it was just the Genentech compounds that evoked this response, or LRRK2 inhibition in general. Plus, he thought it was important to know if this pneumocyte response was reversible. Baptista contacted researchers at Merck Research Laboratories in Boston, Pfizer Inc. in Cambridge, Massachusetts, and Denali in South San Francisco, which had taken over the Genentech program and had since developed its own LRRK2 inhibitors. All three agreed to collaborate. “This was a win-win,” Baptista told Alzforum. “It would have taken a lot longer to conduct this study without the consortium.” This type of collaboration does not happen on its own among pharma companies, he noted.
The researchers chose to study GNE-7915, Pfizer’s PFE-360, and Merck’s MLi-2, because each has a distinct structure and unique off-target effects. These compounds are experimental and are not being tried in the clinic. Baptista and colleagues first tested the three compounds in a 15-day toxicity study in macaques. The three compounds have different potencies, but the researchers aimed for doses that would amount to the same amount of inhibition of LRRK2 phosphorylation at serine 935, a commonly used marker for LRRK2 kinase activity.
For GNE-7915, the positive control, the researchers used a 30 mg/Kg twice-daily dose that previously caused the pneumocyte dysmorphia. [Warning: Arcane details incoming. Hang in there! They will become important later.] This yielded plasma exposures of two to 3.2 times the compound’s IC50, i.e., the concentration that cuts LRRK2 activity in half. For the PFE-360 and MLi-2 compounds, the scientists’ goal was to reach one time and 10 times their IC50 for low and high doses, respectively. Doses calculated on previous PK/PD data worked reasonably well for PFE-360, yielding plasma exposures of 1.0 to 1.2 times and 8.1 to 10.2 times the IC50 for 3 mg/Kg and 6 mg/Kg doses, respectively. For MLi-2, Merck scientists found considerable variability from one macaque to the next, so they erred on the high side to ensure adequate exposure, ending up with 9.5 to 16.9 times and 45.5 to 64.8 times the compound’s IC50 for 15 mg/Kg and 50 mg/Kg doses, respectively. Two male and two female monkeys were treated with each drug and dose combination.

Vacuolation. Large vacuoles (black arrows) appear in the lungs of macaques treated with GNE-7915 (right). They are larger and more numerous than those found in placebo-treated controls (left). [Courtesy of Baptista et al., Science Translational Medicine.]
After 15 days of this, GNE-7915, and high doses of both the Pfizer and Merck drugs, caused lung changes consistent with those seen in the prior Genentech macaque study and in rodent LRRK2 knockouts. Each of the six male and six female monkeys developed large cytoplasmic vacuoles in their type II pneumocytes, which were easily visible with light microscopy (see image above).
The scientists found no such changes in the kidney or brain, though similar levels of drug had reached those organs as it infiltrated the lungs. As for the relevance of the IC50 targets, lungs from monkeys treated with the lower doses of PFE-360 and MLi-2 looked normal, suggesting a dose response.
Further, the effect appeared to be reversible, at least in the case of the Genentech compound. Researchers tested two more male and two more female animals two weeks after halting dosing, and their lungs appeared normal at that point.
What does this mean for clinical trials of LRRK2 inhibitors? “We are absolutely confident that this is an on-target effect, since all compounds induced this same phenotype,” said Baptista.
Does this mean the end for this therapeutic approach, then? Baptista and others say no. Carole Ho from Denali believes these morphological changes do not constitute damage. “Those findings in the lung are not considered adverse,” she told Alzforum. In fact, she thinks the increased lamellae reflect a treatment response. She said that in cell-based studies, failing lysosomal function due to LRRK2 mutations can be rescued by LRRK2 inhibition, which duplicates lysosomal membrane layers. “We believe these changes are part of the therapeutic mechanism,” she said.
For his part, Baptista emphasized that it may be possible to target LRRK2 in the brain without causing these lung effects. “Even with the lowest doses of the Merck compound we were able to completely block LRRK2 kinase activity in the brain, yet those animals did not develop any lung phenotype,” he said.
Importantly, the researchers found that the lungs functioned normally. They put a separate group of macaques through lung tests typically carried out on people. They included measures of elasticity, forced vital capacity, forced expiratory volume, forced expiratory flow, and mean mid-expiratory flow. There was no change from baseline over the course of the 15 daily treatments or 13 days after dosing stopped. “This is not to say there may not be potential on-target effects with other compounds, with higher doses, or with longer duration,” said Baptista. “That will require further study, but that is not what we were setting out to test,” he said. “We wanted to help facilitate early clinical trials.”
Cookson does not believe the lung morphology is a show-stopper, either. “Lung effects give people pause, because, unlike kidney damage, it is not always straightforward to measure, and it is very topical now with biological pneumonia being a serious global health problem,” he said. “The real message here is that a lung effect is something you need to know about and monitor, but that it will reverse when you discontinue treatment.”
How does blocking LRRK2 enlarge pneumocyte vacuoles? This seems related to the kinase’s role in lysosomal function. Seen in the transmission electron microscope, vacuoles in treated animals were filled with the lysosomal lamellae that typically carry and extrude surfactant.
On these findings Matthias Ochs, of the Charité, Berlin, offered a word of caution. He thinks these structures may have been poorly preserved in this study, because special phospholipid-retention protocols were not used and surfactant may have been lost. “What one cannot see, one cannot assess,” Ochs wrote (see comment below). He also considers a qualitative analysis insufficient. “In order to assess the degree of changes, a formal quantitative approach based on stereology and following the official research policy statement of the American Thoracic Society/European Respiratory Society is necessary,” he wrote (Hsia et al., 2010). Ochs helped establish that policy.
Baptista and colleagues report no change in surfactant levels in the lungs of the treated animals. This is in keeping with the absence of a functional effect. The authors did find a reduction in urinary di-22:6-bis(monoacylglycerol) phosphate in monkeys treated with the high dose of PFE-360, or the Genentech compound. This drop was also found in the prior Genentech study and in mouse LRRK2 knockouts. Di-22:6-BMP lines the inner membranes of late endosomes. Plasma levels of this lipid increase in some lysosomal storage disorders and in people who carry the G2019S LRRK2 variant (Alcalay et al., 2020).
The researchers found no change in BMP in the macaques’ plasma, lungs, kidneys, or brain, but levels were highly variable in all four types of organ sample. “It is not clear where the urinary BMP comes from, but the reduction is a consistent preclinical finding that is replicating in the right direction,” Baptista said. In the GNE-7915-treated macaques, urinary BMP had returned to normal two weeks after dosing stopped, again suggesting a reversible effect. Baptista thinks urinary BMP could be a useful biomarker to select for populations to test with LRRK2 inhibitors.
Only Denali is currently testing small-molecule LRRK2 inhibitors in clinical trials; Ionis/Biogen are testing an LRRK2 antisense oligonucleotide (see clinicaltrials.gov). Ho told Alzforum that Denali’s two compounds, DNL201 and DNL151, thus far have been tested in more than 100 volunteers and no pulmonary abnormalities were found. “We have conducted rigorous pulmonary testing, including diffusion capacity, a very sensitive test that might be the first to detect any abnormality if there are adverse effects or damage to type II pneumocytes,” she said.
DNL201 has completed a Phase 1B trial. According to Ho, DNL151 has the advantage that patients would need to take it only once a day, not twice a day as is the case for 201. Denali is starting efficacy trials for 201, but continuing Phase 1 of DNL151. “We will probably decide by midyear which to move forward with,” Ho said.
Jie Shen, Brigham and Women’s Hospital, Boston, cautioned against using LRRK2 inhibitors. She reported that knocking out both LRRK2 and its homolog LRRK1 leads to age-dependent dopaminergic neuron loss in mice (Giaime et al., 2017).
Ho believes the Denali compounds block LRRK2 specifically. “We have no evidence they affect LRRK1, or that they cause any loss of dopaminergic neurons in preclinical studies,” Ho said. She also noted that people who carry one copy of an inactive LRRK2 appear to live a normal lifespan, without evidence of kidney or lung disease (Blauwendraat et al., 2018). Denali is aiming to halve LRRK2 kinase activity in clinical trials, since LRRK2 risk variants typically double the enzyme’s activity.—Tom Fagan
References
News Citations
Paper Citations
- Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, Lynch J, Healy DG, Holton JL, Revesz T, Wood NW. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005 Jan 29-Feb 4;365(9457):415-6. PubMed.
- Biskup S, Moore DJ, Rea A, Lorenz-Deperieux B, Coombes CE, Dawson VL, Dawson TM, West AB. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007 Nov 28;8:102. PubMed.
- Sheng Z, Zhang S, Bustos D, Kleinheinz T, Le Pichon CE, Dominguez SL, Solanoy HO, Drummond J, Zhang X, Ding X, Cai F, Song Q, Li X, Yue Z, van der Brug MP, Burdick DJ, Gunzner-Toste J, Chen H, Liu X, Estrada AA, Sweeney ZK, Scearce-Levie K, Moffat JG, Kirkpatrick DS, Zhu H. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci Transl Med. 2012 Dec 12;4(164):164ra161. PubMed.
- Ness D, Ren Z, Gardai S, Sharpnack D, Johnson VJ, Brennan RJ, Brigham EF, Olaharski AJ. Leucine-rich repeat kinase 2 (LRRK2)-deficient rats exhibit renal tubule injury and perturbations in metabolic and immunological homeostasis. PLoS One. 2013;8(6):e66164. Print 2013 PubMed.
- Tong Y, Giaime E, Yamaguchi H, Ichimura T, Liu Y, Si H, Cai H, Bonventre JV, Shen J. Loss of leucine-rich repeat kinase 2 causes age-dependent bi-phasic alterations of the autophagy pathway. Mol Neurodegener. 2012;7:2. PubMed.
- Baptista MA, Dave KD, Frasier MA, Sherer TB, Greeley M, Beck MJ, Varsho JS, Parker GA, Moore C, Churchill MJ, Meshul CK, Fiske BK. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS One. 2013;8(11):e80705. Epub 2013 Nov 14 PubMed.
- Fuji RN, Flagella M, Baca M, Baptista MA, Brodbeck J, Chan BK, Fiske BK, Honigberg L, Jubb AM, Katavolos P, Lee DW, Lewin-Koh SC, Lin T, Liu X, Liu S, Lyssikatos JP, O'Mahony J, Reichelt M, Roose-Girma M, Sheng Z, Sherer T, Smith A, Solon M, Sweeney ZK, Tarrant J, Urkowitz A, Warming S, Yaylaoglu M, Zhang S, Zhu H, Estrada AA, Watts RJ. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med. 2015 Feb 4;7(273):273ra15. PubMed.
- Hsia CC, Hyde DM, Ochs M, Weibel ER, ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010 Feb 15;181(4):394-418. PubMed.
- Alcalay RN, Hsieh F, Tengstrand E, Padmanabhan S, Baptista M, Kehoe C, Narayan S, Boehme AK, Merchant K. Higher Urine bis(Monoacylglycerol)Phosphate Levels in LRRK2 G2019S Mutation Carriers: Implications for Therapeutic Development. Mov Disord. 2020 Jan;35(1):134-141. Epub 2019 Sep 10 PubMed.
- Giaime E, Tong Y, Wagner LK, Yuan Y, Huang G, Shen J. Age-Dependent Dopaminergic Neurodegeneration and Impairment of the Autophagy-Lysosomal Pathway in LRRK-Deficient Mice. Neuron. 2017 Nov 15;96(4):796-807.e6. Epub 2017 Oct 19 PubMed.
- Blauwendraat C, Reed X, Kia DA, Gan-Or Z, Lesage S, Pihlstrøm L, Guerreiro R, Gibbs JR, Sabir M, Ahmed S, Ding J, Alcalay RN, Hassin-Baer S, Pittman AM, Brooks J, Edsall C, Hernandez DG, Chung SJ, Goldwurm S, Toft M, Schulte C, Bras J, Wood NW, Brice A, Morris HR, Scholz SW, Nalls MA, Singleton AB, Cookson MR, COURAGE-PD (Comprehensive Unbiased Risk Factor Assessment for Genetics and Environment in Parkinson’s Disease) Consortium, the French Parkinson’s Disease Consortium, and the International Parkinson’s Disease Genomics Consortium (IPDGC). Frequency of Loss of Function Variants in LRRK2 in Parkinson Disease. JAMA Neurol. 2018 Nov 1;75(11):1416-1422. PubMed.
External Citations
Further Reading
Papers
- Hsia CC, Hyde DM, Ochs M, Weibel ER, ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010 Feb 15;181(4):394-418. PubMed.
Primary Papers
- Baptista MA, Merchant K, Barrett T, Bhargava S, Bryce DK, Ellis JM, Estrada AA, Fell MJ, Fiske BK, Fuji RN, Galatsis P, Henry AG, Hill S, Hirst W, Houle C, Kennedy ME, Liu X, Maddess ML, Markgraf C, Mei H, Meier WA, Needle E, Ploch S, Royer C, Rudolph K, Sharma AK, Stepan A, Steyn S, Trost C, Yin Z, Yu H, Wang X, Sherer TB. LRRK2 inhibitors induce reversible changes in nonhuman primate lungs without measurable pulmonary deficits. Sci Transl Med. 2020 Apr 22;12(540) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Novartis Institutes for BioMedical Research
Mutations in the LRRK2 gene increase its kinase activity and represent one of the most common genetic causes of Parkinson’s disease (PD). However, preliminary studies with LRRK2 inhibitors in nonhuman primates showed changes in lung morphology and questioned the feasibility of their development for therapy in PD. Baptista and colleagues now show very interesting and encouraging data on different LRRK2 inhibitors and their effects on lung changes in nonhuman primates.
They show convincingly that LRRK2 inhibitors exist that can be used at doses that do not induce lung changes and still inhibit LRRK2 kinase activity in the brain. Furthermore, lung changes were reversible, no functional impairments were detected, and there seems to be a certain safety margin appearing. This data is of utmost importance to the PD research field to encourage further investment into the development of LRRK2 inhibitors as a therapeutic avenue for PD.…More
The Michael J. Fox Foundation supported this study, and several pharma companies participated in this important effort to find disease-modifying therapies for PD. This incredible collaboration and fruitful teamwork are remarkable and necessary to tackle, and get closer to, a therapy for devastating complex neurodegenerative diseases such as PD. These kinds of collaborative efforts are highly welcome. They greatly serve humankind, and it would be desirable to see more of these endeavors.
Charité - Universitätsmedizin Berlin
As presented, I am not quite convinced by the findings regarding type II alveolar epithelial cells, and by their interpretation. I’d point out two main points:
1. The quality of the preservation of the content of the lamellar bodies in type II alveolar epithelial cells could be greatly improved. In this paper, it appears poor because of inadequate fixation and processing of samples for electron microscopy. In order to assess lamellar bodies qualitatively and quantitatively, special phospholipid retention protocols have to be used, otherwise the surfactant material is extracted. What one cannot see, one cannot assess.
2. A qualitative analysis is insufficient. To assess the degree of changes, a formal quantitative approach based on stereology, and following the official research policy statement of the ATS/ERS is necessary. This could still be done. It would require adequate representative sampling (systematic uniform random sampling) and measurements (number of alveolar epithelial type II cells per lung and mean size of alveolar epithelial type II cells for assessing hyperplasia/hypertrophy; number of lamellar bodies per alveolar type II cell and mean size of lamellar bodies for assessing intracellular surfactant pool size and distribution; both of these parameters require use of the disector technique).…More
Ottawa Hospital Research Institute
Gain-of-function mutations found in the LRRK2 gene that increase its kinase activity are associated with Parkinson’s disease. Hence, a specific pharmacological inhibition of LRRK2 kinase activity can be an efficient treatment for halting onset and/or development of PD. LRRK2 kinase activity is also responsible for enhanced inflammatory responses to a pro-inflammatory stimulus.
Genetic ablation of LRRK2 kinase activity in murine models of infection does not raise concerns for mounting a proper immune response, suggesting that if there is a way for a specific pharmacological inhibition of LRRK2 kinase activity there should be no weakening of the host immune system.
However, with the COVID-19 pandemic it becomes apparent that any drug that has a potential to cause physiological changes to lungs, even transiently, should be used cautiously—especially in PD patients, who are usually older. Such treatment will likely have to be chronic, if not lifelong. What this will mean for the function of LRRK2 and off-target kinases is not known.…More
While the report—that the lung tissue effects seen with inhibition of LRRK2 are reversible—is encouraging, effects of a life-long treatment are hardly measurable. Not only in terms of lung physiology, but for normal function of the immune system as well.
Work on inhibition of LRRK2 as a treatment for PD is very promising and I expect it to evolve further, for example drug delivery to the brain only, so we can focus at least on one organ when it comes to side effects of LRRK2 inhibition.
Make a Comment
To make a comment you must login or register.