Plasma NfL Goes the Distance in Alzheimer’s
Quick Links
Damaged axons spill their guts, and the debris eventually makes its way into the bloodstream. Now, scientists tracking one such neuron-derived protein are moving closer to a blood test for neurodegeneration in Alzheimer’s disease. In a longitudinal study of people with AD, blood levels of the neurofilament light protein (NfL) rose over time, and the rate of rise paralleled established cerebrospinal fluid and imaging markers of neuron death and advancing cognitive troubles. The work, the first to follow such a large group of people with the common, late-onset form of AD, strengthens the case for blood NfL as an easy and accessible surrogate measure of neurodegeneration in the brain. The study, by Niklas Mattsson, Lund University, Sweden, and Henrik Zetterberg and Kaj Blennow, University of Gothenburg, appeared in the April 22 JAMA Neurology.
- Study measured NfL over time in 1,583 ADNI participants.
- NfL rose with age and in MCI; accelerated in AD.
- Rate of change tracks with other neurodegeneration markers, cognitive loss.
“The results … highlight the potential utility of plasma NfL as a biomarker of neurodegeneration and disease progression,” wrote Michelle Mielke, Mayo Clinic, Rochester, Minnesota, to Alzforum. A blood-based biomarker is particularly attractive as an endpoint for clinical trials and to determine the rate of disease progression in the general population, she said.
The data echo recent findings in people with early onset, familial AD, from Mathias Jucker’s group at University of Tübingen, Germany, and the DIAN researchers at Washington University in St. Louis (Jan 2019 news). Jucker called the new study “very interesting. This supports our study in familial AD and stresses again that NfL is a dynamic biomarker predicting disease progression,” he said.
NfL is not specific to AD. Blood levels increase in several neurodegenerative conditions and after brain injury. Cross-sectional studies found plasma NfL up in people with MCI over cognitively unimpaired people, and higher still in those with AD (Mar 2017 news). High levels of NfL predicted cognitive decline and loss of brain volume over the following years. But researchers lacked a definitive picture of how NfL changed over the course of AD in the same person.
For their longitudinal study, Mattsson and colleagues took advantage of blood samples collected from 1,583 participants as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI). With an average age of 73, the group comprised 401 cognitively unimpaired (CU) volunteers, 855 with mild cognitive impairment (MCI), and 327 with AD dementia, who gave a total of 4,326 plasma samples over a span of up to 11 years. The investigators analyzed plasma NfL using their in-house, ultra-sensitive single-molecule array immunoassay.
In agreement with their previous work, NfL levels started to rise before overt dementia set in. At baseline, cognitively normal people averaged the lowest levels of NfL, the MCI group was higher, and those with AD were highest. In all three groups, NfL increased with age. The CU and MCI groups rose at about the same rate (2.4 and 2.7 ng/L, respectively), while the AD group rose significantly faster (4.9 ng/L/year).
The rate of increase of NfL was linked to amyloid deposition: In the cognitively unimpaired or MCI groups, those with low CSF Aβ42 had a significantly quicker rise in NfL than the amyloid-negative groups. This indicates that NfL may be used to detect incipient neurodegeneration in preclinical AD, Mattsson wrote to Alzforum. That jibes with the DIAN data, where blood NfL started to increase 16 years before the expected onset of dementia (Preische et al., 2019; Weston et al., 2017).
Plasma NfL tracked with a raft of other disease markers. Rising NfL correlated with declining CSF Aβ42 and increasing tau, with shrinking hippocampi and cortex on MRI, with waning glucose metabolism, expanding white-matter lesions, and sinking scores on multiple cognitive tests, all independent of diagnosis. Of CSF biomarkers, Aβ42 was most strongly associated with NFL level longitudinally; among imaging measures, the top association was with hippocampal volume and entorhinal cortex thickness, and with changes in the ADAS-Cog among cognitive measures.
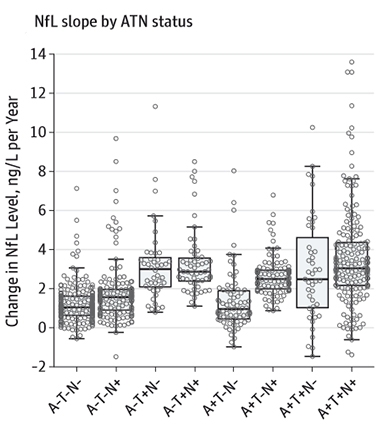
Trouble Rising. Rate of change of NfL picks up in people with positive biomarkers for tau pathology (T+) or neurodegeneration, as indicated by cortical thinning (N+). [Courtesy of Mattsson et al. © 2019 American Medical Association. All rights reserved.]
The investigators also compared plasma NfL to biomarkers in the A/T/N classification scheme (Apr 2018 news). This system defines disease states according to biomarkers for brain amyloid (A), tau pathology (T), and neurodegeneration (N). Current measures of N include FDG-PET glucose uptake, MRI assessments of brain atrophy, or CSF total tau. A recent study held out CSF NfL as an alternative marker for neurodegeneration in this scheme (Nov 2018 news).
What about blood NfL? When Mattson examined NfL in ADNI volunteers binned according to the A/T/N criteria, the N+ subsets had a higher baseline and faster rising NfL than N- groups. Also, T+ people had greater NfL rise. “Tau pathology is closely associated with neurodegeneration, which leads to release of NFL. The response of the NFL slopes to tau positivity support that association,” said Mattsson. Going forward, it will be interesting to compare the changes in NfL to additional aspects of AD not included in this study, such as the spread of tau measured by PET imaging, he said.
Anne Fagan, Washington University, St. Louis, Missouri, said studies in both late-onset and autosomal dominant AD have shown correlations between imaging variables and plasma NfL levels, but the strength of this study is its evaluation of within-person change over time. “As always, this is a terrific study by this group, and very comprehensive. It lays the groundwork for future studies to define cutoffs and evaluate the performance of NfL as an indicator of neurodegeneration as defined by the proposed ATN framework,” she said.—Pat McCaffrey
References
News Citations
- Neurofilament in Blood Foretells Early Onset Alzheimer’s
- Blood Neurofilament Light a Promising Biomarker for Alzheimer’s?
- New Definition of Alzheimer’s Hinges on Biology, Not Symptoms
- Is NfL the New 'N' in A/T/N Classification of Alzheimer’s?
Paper Citations
- Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, Gräber S, Kuder-Buletta E, LaFougere C, Laske C, Vöglein J, Levin J, Masters CL, Martins R, Schofield PR, Rossor MN, Graff-Radford NR, Salloway S, Ghetti B, Ringman JM, Noble JM, Chhatwal J, Goate AM, Benzinger TL, Morris JC, Bateman RJ, Wang G, Fagan AM, McDade EM, Gordon BA, Jucker M, Dominantly Inherited Alzheimer Network. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019 Feb;25(2):277-283. Epub 2019 Jan 21 PubMed.
- Weston PS, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, Druyeh R, Malone IB, Ahsan RL, Pemberton H, Klimova J, Mead S, Blennow K, Rossor MN, Schott JM, Zetterberg H, Fox NC. Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology. 2017 Nov 21;89(21):2167-2175. Epub 2017 Oct 25 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2019 Jul 1;76(7):791-799. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University in St. Louis
Before we can say what utility NfL may have, we need to understand how levels of NfL discriminate between diseases states in a cross-sectional, one-off manner, as well as how it behaves over time. Understanding such a longitudinal change is critical for any biomarker that could be considered for a clinical trial.
The strengths of the current study are the large population, which is impressive, and the sheer amount of longitudinal data. In this large sample, the authors clearly demonstrate that, at the group level, rates of change in NfL increase with greater cognitive impairment as well as with worse biological profiles of Aβ, tau, and neurodegeneration. This result is consistent with what …More
As to the clinical utility of NfL at an individual level, I think the jury is still out on that one. Although the current paper finds significant differences, there is a large degree of overlap in NfL rates of change between groups. Also, the vast majority of individuals in the study are showing positive annual rate of change in NfL values. This suggests that there could be a background amount of change in NfL associated with aging, making it problematic to pick up disease-related effects.
Still, the fact that NfL can be detected in blood makes it an excellent candidate biomarker. Such a low-cost measure, even with its limitations, could be a boon to noninvasively studying injury in the brain across neurodegenerative diseases.
UCL
This is an important and timely study, which examines longitudinal plasma NfL data on large numbers of individuals at different stages of sporadic AD.
The findings appear consistent with what we and others have found previously in autosomal-dominant AD, with the concentration of NfL in blood beginning to rise early in the disease, and continuing to rise progressively thereafter, with plasma NfL appearing to reflect both disease severity and intensity. As the authors discuss, the potential availability of such a measure from a simple blood test, as opposed to requiring either CSF sampling or expensive imaging techniques, would be extremely valuble.
However, although statistically significantly …More
Therefore at the current time, while showing promise as a potential measure in trials to compare different groups over time, it remains unclear exactly how useful longitudinal measurement of plasma NfL may be when applied to individual patients to inform individual patient treatment decisions. Further studies will hopefully help to answer this question.
The study also reinforces what we already knew in terms of NfL measurement not being specific to AD, as it was strongly associated with amyloid-negative neurodegeneration also. Therefore I think, rather than being used diagnostically, its main utility in AD is likely to be in monitoring and assessment to identify those individuals most at risk of clinical decline in the near future, and in tracking subsequent neurodegenerative change.
Mayo Clinic
This paper is an important contribution to the field. Although previous studies examined plasma NfL and Alzheimer’s disease, there is a dearth of studies with serial measures of plasma NfL. Therefore, it was not known whether plasma NfL serially tracked with ongoing neurodegeneration over the clinical AD spectrum. The results of the current study highlight the potential utility of plasma NfL as a biomarker of neurodegeneration and disease progression. Such a biomarker, especially one that is blood-based, is particularly attractive as an endpoint for clinical trials and to determine rate of disease progression in the general population.
Although the results of this study are quite promising, …More
For example, many participants with vascular disease were excluded from ADNI. This is important because NfL (plasma and CSF) has been shown to also be elevated in vascular dementia and stroke patients. It will be important to replicate the current study in a more general population that considers vascular pathology and vascular risk factors. It also is not well understood what other factors might affect plasma NfL levels, especially because it is thought to be a nonspecific neurodegeneration marker. This research will also be needed before plasma NfL can be used clinically.
Make a Comment
To make a comment you must login or register.