Phosphorylation of FUS Does Away with Droplets
Quick Links
Cytoplasmic aggregates of the FUS protein have a hand in amyotrophic lateral sclerosis (ALS), and the protein has been spotted loitering in viscous granules thought to breed such cellular inclusions. Now, a study published in the EMBO Journal on August 8 describes a potential “off switch” that steers FUS away from this toxic crowd. Researchers led by Nicolas Fawzi at Brown University in Providence, Rhode Island, report that phosphorylation of FUS within its low-complexity (LC) domain—a region the protein uses to hook up with others of its ilk—repelled other FUS proteins and discouraged the formation of liquid droplets. Mutants of FUS that mimic phosphorylated forms aggregated less in mammalian cells, and were less toxic to yeast cells than wild-type proteins.
While the physiological significance of FUS phosphorylation and its role in disease are far from fully understood, Fawzi proposed that equipping FUS with more of this common phosphate adornment could pose a viable therapeutic strategy.
The findings add further support to the idea that LC domains play a key role in liquid phase transitions that form granules inside cells, commented Benjamin Wolozin of Boston University. “More importantly, they also highlight how cells can use post-translational modifications to regulate those transitions,” Wolozin said.
FUS is among several neurodegenerative-disease-related proteins spotted in intracellular granules. These membrane-less organelles gain their structure through liquid-liquid phase separation (LLPS), a phenomenon often facilitated via associations between LC domains. FUS, an RNA-binding protein, has both LCs and RNA-binding regions, and has been found within RNA granules (see Oct 2015 news). FUS mutations that lead to ALS promote the formation of such granules, and researchers have proposed the close quarters in the granules facilitate toxic aggregation and/or other cellular woes (Wolozin, 2014; May 2016 news; Oct 2016 news).
This is not the first time phosphorylation of FUS’s LC domain has been reported. Twelve conserved serine-threonine motifs in FUS’s LC domain are recognition sites for DNA-PK, a kinase involved in the DNA damage response. A 2008 study reported that five of these motifs were indeed phosphorylated by the kinase (Gardiner et al., 2008). Steve McKnight’s lab later confirmed these five, and reported that phosphorylation reduced the protein’s ability to interlock with hydrogels of FUS fibrils, suggesting that this post-translational modification could dispel cellular granules (Han et al., 2012).

Clumpy to Cloudy.
In yeast cells, wild-type FUS (green) appears in distinct puncta (top), but disperses as more phosphomimetic residues are substituted in the LC domain (middle, bottom). [Courtesy of Monahan et al., EMBO Journal, 2017.]
In the present paper, first author Zachary Monahan and colleagues set out to map FUS phosphorylation sites, and to characterize more fully how phosphorylation affected aggregation, LLPS, and toxicity exerted by the protein. The researchers suspected that more than five of the serine-threonine motifs in FUS’s LC could be phosphorylated by DNA-PK. This is because FUS’s LC domain is devoid of charged amino acids, which makes it a tricky target for mass spectrometry used in other studies. The researchers therefore used solution nuclear magnetic resonance. NMR showed that the FUS-LC is indeed phosphorylated by DNA-PK on all 12 sites in vitro. This finding extended to FUS expressed in cells, where provoking DNA-PK activation via DNA damage triggered extensive phosphorylation of the FUS LC domain.
Monahan next asked how phosphorylation of FUS’s LC domain would affect liquid phase separation and aggregation. The researchers found that, given a day in a dish, the wild-type FUS LC domain readily formed droplets and even fibrous aggregates. However, mixing FUS-LC with DNA-PK or using a mutant form of FUS-LC containing phosphomimetic residues at all 12 phosphorylation sites prevented both droplet and aggregate formation.
Based on these and a slew of biophysical experiments, the researchers proposed a model in which FUS proteins associate with one another via their LC domains. When phosphorylated, the LC domains repel each other, causing dispersal of liquid droplets and preventing aggregation. Cellular experiments supported that hypothesis, as both yeast and mammalian cells expressing full-length FUS with six or 12 phosphomimetic residues formed less punctate, cytoplasmic inclusions than did cells expressing FUS with a normal LC domain. In yeast cells, expression of wild-type FUS slowed growth more than did phosphomimetic forms.
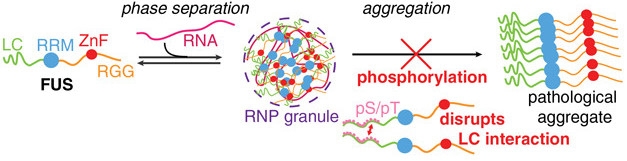
FUS Repulsion. Interactions between its LC domains make FUS clump into RNA granules, which could facilitate pathological aggregate formation. Phosphorylation repels LC domains, preventing aggregation. [Courtesy of Monahan et al., EMBO Journal, 2017.]
Compared with the way FUS LC domains interact in a dish, the picture is likely to be far more complicated within cells, the authors acknowledged. There, many different proteins and RNAs make up granules.
Wolozin added that other RNA binding proteins, such as TDP-43, might be regulated differently than FUS. For its part, FUS is known to respond to DNA damage, so the fact that its aggregation state, and therefore its function, is regulated by DNA-PK makes biological sense, he said (Wang et al., 2013). Other phase-transitioning proteins are likely regulated by post-translational modifications that fit with their functional modus operandi, he added.
Fawzi and colleagues proposed boosting FUS phosphorylation within the LC domain as a therapeutic strategy. Because activating DNA-PK might have unintended consequences, the researchers are looking for other kinases that might do the job.—Jessica Shugart
References
News Citations
- Do Membraneless Organelles Host Fibril Nucleation?
- Stress Granule Protein Entwines and Misfolds Tau
- ALS Research ‘Gels’ as Studies Tie Disparate Genetic Factors Together
- Paper Alert: FUS a Fixer of Damaged DNA
Paper Citations
- Wolozin B. Physiological protein aggregation run amuck: stress granules and the genesis of neurodegenerative disease. Discov Med. 2014 Jan;17(91):47-52. PubMed.
- Gardiner M, Toth R, Vandermoere F, Morrice NA, Rouse J. Identification and characterization of FUS/TLS as a new target of ATM. Biochem J. 2008 Oct 15;415(2):297-307. PubMed.
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, McKnight SL. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012 May 11;149(4):768-79. PubMed.
Further Reading
Primary Papers
- Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, O'Meally R, Dignon GL, Conicella AE, Zheng W, Best RB, Cole RN, Mittal J, Shewmaker F, Fawzi NL. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 2017 Oct 16;36(20):2951-2967. Epub 2017 Aug 8 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.