Not Just Aβ—Glymphatic Flow Clears Tau, Too, Slowing Its Aggregation
Quick Links
The brain rinses away Aβ during sleep, powered by glymphatic flow from arteries to veins. In today’s Journal of Experimental Medicine, researchers led by Takeshi Iwatsubo at the University of Tokyo present evidence that this process clears extracellular tau as well, and that it can attenuate neurodegeneration. Tauopathy mice lacking aquaporin 4 (AQP4), which have impaired glymphatic clearance, accumulated more insoluble p-tau in the brain and lost more neurons with age than did those with a working glymphatic system. The clearance may wash away aggregated tau seeds that can spread pathology, Iwatsubo suggested.
- Reducing glymphatic flow slowed the clearance of extracellular tau from mouse brain.
- In tauopathy mice, this caused faster accumulation of insoluble p-tau in neurons.
- Neuron loss accelerated.
“This carefully conducted research identifies AQP4 as an important molecule for extracellular tau transport from brain to CSF via glymphatic system,” noted Tsuneya Ikezu at the Mayo Clinic in Jacksonville, Florida. The data hint that enhancing AQP4 function or increasing its expression might help prevent tau buildup, Ikezu said.
Glymphatic flow was first described by Maiken Nedergaard at the University of Rochester Medical Center, New York, and Jeffrey Iliff, now at the University of Washington in Seattle. They reported that AQP4 water channels on astrocyte endfeet around cerebral blood vessels drive the movement of interstitial fluid along arterioles and veins. This current picks up solutes from the parenchymal extracellular space and washes them into the perivascular space around veins, where they flow from the brain (Aug 2012 news). Later studies found that this drainage, which increases during sleep, helps clear Aβ (Mar 2013 news; Jan 2014 webinar). The efficiency of the system wanes with age, after strokes, and in the Alzheimer’s brain (May 2014 news; Mar 2017 news; Dec 2016 news).
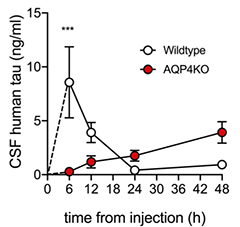
Sluggish Clearance. Injected into wild-type mouse brain, human tau peaks in CSF within six hours (white), and washes out by 24. In AQP4 knockouts, it is still rising after 48 hours (red). [Courtesy of Ishida et al., JEM.]
Unlike Aβ, tau is predominantly an intracellular protein, though some is released into the extracellular space, and its level there spikes during wakefulness and sleep deprivation (Jan 2019 news). This led Iwatsubo to wonder whether tau was also cleared by glymph.
To test this, joint first authors Kazuhisa Ishida and Kaoru Yamada injected fluorescently labeled human tau into the striata of wild-type and AQP4 knockout mice. In wild-types, tau diffused through the brain within 12 hours, and was mostly cleared from parenchyma by 48. In the knockouts, by contrast, tau spread more slowly and not as far, and most of it remained in brain tissue at 48 hours.
The brain’s glymphatic system connects to the flow of cerebrospinal fluid. CSF circulates through the brain, entering along arteries and being carried to veins by glymphatic flow before exiting again into the subarachnoid space, carrying solutes with it. These solutes are absorbed by dural lymphatic vessels and transported to deep cervical lymph nodes (Da Mesquita et al., 2018). Thus, the speed with which a molecule flows through the CSF system helps assess the efficiency of glymphatic clearance. In wild-type mice, tau injected into the brain quickly appeared in the CSF, peaking by six hours and nearly gone by 24. In AQP4 knockouts, CSF tau rose slowly, and was still low at 48 hours (see image above). In a separate experiment, the authors injected labeled tau directly into the subarachnoid space, then measured how quickly it arrived in cervical lymph nodes. In wild-type mice, it showed up within an hour after injection, but in knockouts, almost none appeared.
What effect does this slow clearance have on the brain? The authors crossed AQP4 knockouts with PS19 tauopathy mice, which express human tau with the P301S mutation. At six months of age the AQP4-negtive mice had more paired helical filaments of p-tau in the hippocampus and about three times as much total tau in CSF as did PS19 controls. By nine months, the AQP4 knockouts had accumulated about twice as much insoluble p-tau in the hippocampus, midbrain, and cortex as controls. Their cortices were 25 percent thinner, and they had about 20 percent fewer cortical neurons than controls. Altogether, the data suggested that slowing tau clearance triggered neurodegeneration.
Does insoluble tau double because seeds trapped in the interstitial fluid spread to nearby neurons, coaxing their soluble tau to aggregate? The authors will test this idea by injecting labeled tau seeds into wild-type and AQP4 knockout mice, then tracking what happens to them. An alternate possibility is that intracellular and extracellular tau are in equilibrium, so that an excess of the latter leads to buildup of the former, which is then more likely to aggregate, Iwatsubo and Yamada wrote.
“The impact of AQP4 deletion on tau pathology, however, is somewhat modest, and some alternative tau clearance pathway may play a compensatory role,” Ikezu noted. He suggested analyzing the transcriptome of AQP4 knockouts to find gene-expression changes that might help clear tau.
Meanwhile, Iwatsubo plans to examine how plaques, tangles, or neuroinflammation change glymphatic clearance. Intriguingly, a previous study of the rTg4510 tauopathy model found AQP4 was depleted around blood vessels in the brain and tau clearance was slowed, hinting at a possible negative feedback loop between tangle accumulation and impaired clearance (Harrison et al., 2020).
The findings add more weight to the old stricture to get a good night’s rest, since glymphatic flow surges during sleep. Indeed, a recent study linked better sleep quality and duration in older adults with both a stronger glymphatic flow and better scores on tests of language and delayed recall (Siow et al., 2022). Whether there might be therapeutic ways to improve glymphatic clearance is less clear. Iwatsubo noted the existence of at least one AQP4 inhibitor, TGN-020, and one enhancer, TGN-073, that affect glymphatic flow (Huber et al., 2009; Huber et al., 2018). However, these are experimental tools that have not been tested in people.—Madolyn Bowman Rogers
References
News Citations
- Brain Drain—“Glymphatic” Pathway Clears Aβ, Requires Water Channel
- Spinal Fluid Flush: Visualizing the Brain Drain With MRI
- Glymphatic Flow, Sleep, microRNA Are Frontiers in Alzheimer’s Research
- Mini Strokes Cause Mega Problems for Brain Cleansing
- Dearth of Water Channels a Sign of ‘Glymphatic’ Breakdown in Alzheimer’s?
- Another Reason to Catch Some Zzzs: Sleep Regulates Tau Release
Webinar Citations
Research Models Citations
Paper Citations
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018 Aug;560(7717):185-191. Epub 2018 Jul 25 PubMed.
- Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, Ahmed Z, Fisher A, Meftah S, Murray TK, Ottersen OP, Nagelhus EA, O'Neill MJ, Wells JA, Lythgoe MF. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020 Aug 1;143(8):2576-2593. PubMed.
- Siow TY, Toh CH, Hsu JL, Liu GH, Lee SH, Chen NH, Fu CJ, Castillo M, Fang JT. Association of Sleep, Neuropsychological Performance, and Gray Matter Volume With Glymphatic Function in Community-Dwelling Older Adults. Neurology. 2022 Feb 22;98(8):e829-e838. Epub 2021 Dec 14 PubMed.
- Huber VJ, Tsujita M, Nakada T. Identification of aquaporin 4 inhibitors using in vitro and in silico methods. Bioorg Med Chem. 2009 Jan 1;17(1):411-7. Epub 2008 Jan 7 PubMed.
- Huber VJ, Igarashi H, Ueki S, Kwee IL, Nakada T. Aquaporin-4 facilitator TGN-073 promotes interstitial fluid circulation within the blood-brain barrier: [17O]H2O JJVCPE MRI study. Neuroreport. 2018 Jun 13;29(9):697-703. PubMed.
Further Reading
Primary Papers
- Ishida K, Yamada K, Nishiyama R, Hashimoto T, Nishida I, Abe Y, Yasui M, Iwatsubo T. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J Exp Med. 2022 Mar 7;219(3) Epub 2022 Feb 25 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University in St. Louis, School of Medicine
Washington University of St. Louis School of Medicine
This paper reports that extracellular tau is cleared by the glymphatic system, and that loss of aquaporin 4 (AQP4)-driven fluid flow accelerates tau accumulation and neurodegeneration in a mouse model of tauopathy. In this elegant study, the authors replicate earlier findings from other groups using Aβ (Iliff et al., 2012; Xu et al., 2015) and demonstrate that AQP4-driven fluid flow through the brain is needed to wash out the brain parenchyma from waste products.
The paper further suggests that proper fluid flow in the brain is needed for the draining function of the brain-covering, dural lymphatics that pick-up CSF and drain it into the deep cervical lymph nodes, thereby contributing to the removal of waste products from the brain (Louveau et al., 2015; Aspelund et al., 2015; Ahn et al., 2019). Ishida and colleagues nicely show that CSF-injected tau in AQP4 KO mice is retained in the CSF, does not reach the deep cervical lymph nodes, and is not influxed as efficiently into the brain parenchyma as it is in wild-type mice.…More
The glymphatic system is compromised in the awake state, in aging, and after brain injuries such as ischemic stroke or subarachnoid hemorrhage (Gaberel et al., 2014), which are all known risk factors for Alzheimer’s disease and other dementias. Interestingly, glymphatic perfusion after subarachnoid hemorrhage could be improved by intracerebroventricular injection of tissue-type plasminogen activator (Gaberel et al., 2014), which suggests that the system is targetable. Future research is needed to find out if, and how, we can employ these routes to enhance brain perfusion and prevent the accumulation of neurotoxic waste in proteinopathies as seen in many neurodegenerative diseases.
The glymphatic system is not alone in mediating brain clearance. AQP4-driven fluid flow through the brain is required for the distribution of extracellular solutes throughout the brain and into the CSF space, and the cells in the brain parenchyma, blood vessels, and dural lymphatics subsequently eliminate waste proteins.
References:
Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, Jeong Y, Park SH, Koh GY. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019 Jul 24; PubMed.
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015 Jun 29;212(7):991-9. Epub 2015 Jun 15 PubMed.
Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis?. Stroke. 2014 Oct;45(10):3092-6. Epub 2014 Sep 4 PubMed.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012 Aug 15;4(147):147ra111. PubMed.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 Jul 16;523(7560):337-41. Epub 2015 Jun 1 PubMed.
Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, Xiao M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015 Nov 2;10:58. PubMed.
Mayo Clinic
This study by Ishida et al. corroborates the important role of the brain perivascular routes, known as the glymphatic system (Iliff et al., 2012; Xie et al., 2013), for the clearance of extracellular tau. As others have shown in the past, defects in aquaporin 4 (AQP4) lead to impaired glymphatic function, and to the retainment of toxic extracellular molecules in the brain’s interstitium, such as the aggregation-prone Aβ (Peng et al., 2016; Xu et al., 2015). In this new study, Ishida et al. show that the same applies to extracellular tau. The present study also shows that deficiency in AQP4 aggravates tau hyperphosphorylation and neurodegeneration in a model of tauopathy.
I find it very interesting that the authors show that drainage of tau from the cerebrospinal fluid into the deep cervical lymph nodes is severely compromised in mice that lack AQP4. This is somewhat puzzling, because the process of CSF drainage into the deep cervical lymph nodes is solely mediated by the lymphatic vasculature, namely the meningeal lymphatics (Louveau et al., 2015; das Neves et al., 2021). It was recently shown that reduced meningeal lymphatic drainage might also contribute to defects in perivascular circulation of CSF through the glymphatic system, and to the buildup of Aβ plaques in the brain (Da Mesquita et al., 2018; Da Mesquita et al., 2021). Moreover, mice that lack a meningeal lymphatic vascular system also present decreased drainage of tau from the brain into the deep cervical lymph nodes (Patel et al., 2019). …More
Taking into consideration that the authors used a constitutive Aqp4-/- mouse line, it will be important to investigate whether there are defects in meningeal lymphatic vasculature in this Aqp4-/- mouse model, developmental or otherwise, that might explain such outcomes.
It will also be essential to understand whether the clearance of extracellular Aβ or tau in Aqp4-/- mice, which present constitutive defects in glymphatics, can be therapeutically boosted by treating mice with the pro-lymphangiogenic vascular endothelial growth factor C, which acts primarily on the meningeal lymphatic vessels draining the central nervous system.
Overall, this is very interesting work that broadens our understanding about the importance of the glymphatic and lymphatic systems for extracellular tau clearance from the brain and might prove to be of great therapeutic relevance for Alzheimer’s disease, frontotemporal dementia, and other neurodegenerative disorders afflicted by evident brain tauopathy.
References:
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012 Aug 15;4(147):147ra111. PubMed.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013 Oct 18;342(6156):373-7. PubMed.
Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol Dis. 2016 Sep;93:215-25. Epub 2016 May 24 PubMed.
Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, Xiao M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015 Nov 2;10:58. PubMed.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 Jul 16;523(7560):337-41. Epub 2015 Jun 1 PubMed.
das Neves SP, Delivanoglou N, Da Mesquita S. CNS-Draining Meningeal Lymphatic Vasculature: Roles, Conundrums and Future Challenges. Front Pharmacol. 2021;12:655052. Epub 2021 Apr 28 PubMed.
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018 Aug;560(7717):185-191. Epub 2018 Jul 25 PubMed.
Da Mesquita S, Papadopoulos Z, Dykstra T, Brase L, Farias FG, Wall M, Jiang H, Kodira CD, de Lima KA, Herz J, Louveau A, Goldman DH, Salvador AF, Onengut-Gumuscu S, Farber E, Dabhi N, Kennedy T, Milam MG, Baker W, Smirnov I, Rich SS, Dominantly Inherited Alzheimer Network, Benitez BA, Karch CM, Perrin RJ, Farlow M, Chhatwal JP, Holtzman DM, Cruchaga C, Harari O, Kipnis J. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature. 2021 May;593(7858):255-260. Epub 2021 Apr 28 PubMed.
Patel TK, Habimana-Griffin L, Gao X, Xu B, Achilefu S, Alitalo K, McKee CA, Sheehan PW, Musiek ES, Xiong C, Coble D, Holtzman DM. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener. 2019 Feb 27;14(1):11. PubMed.
Make a Comment
To make a comment you must login or register.