In NFL Players, Brain Inflammation May Persist Years After Head Trauma
Quick Links
Research has shown that sports-related head injuries lead to future amyloid and tau pathology, as well as a higher risk of dementia and neuropsychiatric symptoms. However, the link between traumatic brain injury (TBI) and these ensuing problems is unclear. Could inflammation play a role? Scientists led by Martin Pomper, Johns Hopkins Medical Institutions, Baltimore, report online in the November 28 JAMA Neurology that NFL players’ brains are replete with activated glial cells even without obvious neuropsychiatric problems. This finding suggests that neuroinflammation could be a marker for problems down the road.
“Overall, this study adds to the accumulating evidence that microglia activation is an early response to the repetitive head trauma that occurs during football play,” wrote Ann McKee, Boston University, to Alzforum.
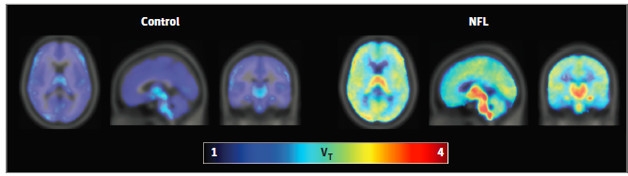
Concussive Impact. NFL players (right) bind more TSPO ligand than matched controls (left), suggesting glial cells are activated. [Courtesy of JAMA Neurology, © 2016 American Medical Association. All rights reserved.]
Prior PET imaging studies with a ligand that binds a microglial transporter protein, called TSPO, suggest that these cells kick into high gear in people who sustain a single severe blow to the head, and that this may be associated with cognitive impairment (Ramlackhansingh et al., 2011). Researchers theorize that after brain injury, chronic inflammation contributes to cellular and structural damage, leading to depression and cognitive decline (Witcher et al., 2015). However, few reports have examined whether this plays out in the brains of athletes who experience repeated mild concussions. Previously, McKee and colleagues reported an abundance of activated microglia in deceased football players compared with controls (Cherry et al., 2016). This occurred most prominently in old athletes decades after retirement, but cropped up even in young players. Using PET, Pomper and colleagues found evidence for microglial activation in older former NFL players (Coughlin et al., 2015). However, these athletes were at increased risk for inflammation due to cardiovascular disease and other problems of aging. To eliminate those confounding factors, the researchers decided to repeat the study in younger players.
To image activated glia, the authors used a PET ligand called [11C]DAP-713. This agent also binds to TSPO, but with greater specificity than some of the early TSPO PET ligands. First author Jennifer Coughlin and colleagues scanned the brains of 14 NFL players—four active and 10 who had retired within the last 12 years—at an average of seven years since their last concussion. One player’s last concussion was 21 years prior, while another was concussed just a year before the scan. Coughlin also scanned 16 active controls matched for age, sex, education, and body mass index, and who had no history of TBI. The average ages of players and controls were 31 and 28 years, respectively. In addition, the scientists conducted neuropsychological tests, structural magnetic resonance imaging (MRI), and diffusion tensor imaging (DTI), which measures the movement of water in brain tissue to estimate damage to white matter.
PET scans revealed that glial cells were indeed more active in these young NFL players compared to controls. Their brains lit up with DAP-713, especially in the bilateral hippocampus, parahippocampal cortex, and supramarginal gyrus, as well as the left entorhinal cortex and temporal pole (see image above). In contrast, there were no significant differences in brain volume or neuropsychological measures of learning, memory, or depression between athletes and controls. Only slight white matter changes appeared in NFL players’ DTI scans, indicating damage in the right posterior thalamic radiation and left anterior corona radiata.
The data suggest that inflammation occurs in players who experience repeated head injuries, and that glial cells can remain revved up for years after injury, said Coughlin. Longitudinal studies will be required to examine whether TSPO binding predicts TBI-related complications, wrote Kristina Witcher and Jonathan Godbout of Ohio State University, Columbus, in an accompanying editorial. Since neuropsychiatric complications and neurodegenerative diseases appear much later, such a finding could open a window of opportunity for preventative anti-inflammatory treatment, they wrote.
Anna-Leena Sirén, University of Würzburg, Germany, pointed out that brain areas relevant for cognitive function seemed to strongly take up the PET ligand in this study (see full comment below). Chronic neuroinflammation after sport-related injuries may drive neurodegeneration there, since activated microglia can be involved in synaptic remodeling and contribute to the spread of tau pathology, she noted (Vasek et al., 2016; Maphis et al., 2015).
Researchers are still unsure what TSPO activation means physiologically, since the ligand does not distinguish between different forms of activated microglia. Scientists believe some of these cells are harmful, spewing out inflammatory chemicals, while others may protect by devouring debris caused by injury. Whichever DAP-713 labels, it is clear the signal marks spots of neuronal injury and repair, said Coughlin. DAP-713 may also pinpoint glia that are primed to turn inflammatory after a second insult, such as another brain injury, as has been reported in animals (Rowe et al., 2016). TSPO also cannot distinguish between activated microglia and reactive astrocytes. McKee noted that her recent postmortem study found the former, not the latter, in deceased football players
Coughlin and colleagues aim to expand this study by imaging more players and controls over time, while measuring fluid biomarkers as well. This may reveal how inflammation ties in with neuropsychiatric symptoms. “We need to better understand the [innate] immune response to inform therapeutic strategies, such as which anti-inflammatory drugs might help these players,” she said. More research is also needed before TSPO-PET becomes a routine part of post-concussion monitoring or care management, she said.—Gwyneth Dickey Zakaib
References
Paper Citations
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011 Sep;70(3):374-83. PubMed.
- Witcher KG, Eiferman DS, Godbout JP. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 2015 Oct;38(10):609-20. PubMed.
- Cherry JD, Tripodis Y, Alvarez VE, Huber B, Kiernan PT, Daneshvar DH, Mez J, Montenigro PH, Solomon TM, Alosco ML, Stern RA, McKee AC, Stein TD. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2016 Oct 28;4(1):112. PubMed.
- Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen S, Airan R, Kim PK, Adams AV, Garcia C, Higgs C, Sair HI, Sawa A, Smith G, Lyketsos CG, Caffo B, Kassiou M, Guilarte TR, Pomper MG. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol Dis. 2015 Feb;74:58-65. Epub 2014 Nov 7 PubMed.
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, Funk K, DeMasters BK, Jiang X, Bowen JR, Mennerick S, Robinson JK, Garbow JR, Tyler KL, Suthar MS, Schmidt RE, Stevens B, Klein RS. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016 Jun 22;534(7608):538-43. PubMed.
- Maphis N, Xu G, Kokiko-Cochran ON, Jiang S, Cardona A, Ransohoff RM, Lamb BT, Bhaskar K. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015 Jun;138(Pt 6):1738-55. Epub 2015 Mar 31 PubMed.
- Rowe RK, Ellis GI, Harrison JL, Bachstetter AD, Corder GF, Van Eldik LJ, Taylor BK, Marti F, Lifshitz J. Diffuse traumatic brain injury induces prolonged immune dysregulation and potentiates hyperalgesia following a peripheral immune challenge. Mol Pain. 2016;12 Print 2016 PubMed.
Further Reading
Papers
- Di Virgilio TG, Hunter A, Wilson L, Stewart W, Goodall S, Howatson G, Donaldson DI, Ietswaart M. Evidence for Acute Electrophysiological and Cognitive Changes Following Routine Soccer Heading. EBioMedicine. 2016 Nov;13:66-71. Epub 2016 Oct 23 PubMed.
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, Crawford F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol. 2014 Feb;75(2):241-54. Epub 2014 Feb 20 PubMed.
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry. 2014 Oct 1;76(7):575-84. Epub 2013 Oct 25 PubMed.
News
- Axon Damage May Hinder Recovery from Concussion, Spark Neurodegeneration
- Brain Trauma Linked to Parkinson’s, Not Alzheimer’s
- Traumatic Brain Injury: Aβ Ensues, but Not Quite Alzheimer’s
- Cognitive Decline in Young Football Player Tied to Extensive Brain Damage
- Axon Damage May Hinder Recovery from Concussion, Spark Neurodegeneration
- CTE: Trauma Triggers Tauopathy Progression
- In Former Footballers, MRI Links Cognitive Problems to Axon Damage
- Dementia Four Times More Likely in Pro Football Players
Primary Papers
- Coughlin JM, Wang Y, Minn I, Bienko N, Ambinder EB, Xu X, Peters ME, Dougherty JW, Vranesic M, Koo SM, Ahn HH, Lee M, Cottrell C, Sair HI, Sawa A, Munro CA, Nowinski CJ, Dannals RF, Lyketsos CG, Kassiou M, Smith G, Caffo B, Mori S, Guilarte TR, Pomper MG. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017 Jan 1;74(1):67-74. PubMed.
- Witcher KG, Godbout JP. Can Sustained Glia-Mediated Brain Inflammation After Repeated Concussive Brain Injury Be Detected In Vivo?. JAMA Neurol. 2016 Nov 28; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
How chronic traumatic brain injury leads to dementia and increases the risk for neurodegenerative disease is not known. This study by Coughlin et al. provides evidence for the role of chronic inflammation, perhaps already early in these processes, by demonstrating profound chronic activation of microglia after mild sport-related head injuries in young football players. As stated in the accompanying commentary, increased TSPO expression might not be direct proof of a chronic proinflammatory state of microglia, but there are robust experimental data showing increased binding of TSPO ligands in activated proinflammatory microglia and a sustained microgliosis in rodent models of brain trauma.
Importantly, Coughlin et al. report that brain areas relevant for cognitive function seemed to be strongly affected. Since activated microglia can be involved in synaptic remodeling (Vasek et al., 2016) contribute to spreading of tau pathology (Maphis et al., 2015), the chronic neuroinflammatory response after sport-related injuries may be the driver of neurodegeneration. As new noninvasive PET-imaging ligands for monitoring of tau pathology become available (Barrio et al., 2015), it would be interesting to further examine tau pathology and microglia activation in the course of mild sport-related head injuries.…More
References:
Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, Funk K, DeMasters BK, Jiang X, Bowen JR, Mennerick S, Robinson JK, Garbow JR, Tyler KL, Suthar MS, Schmidt RE, Stevens B, Klein RS. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016 Jun 22;534(7608):538-43. PubMed.
Maphis N, Xu G, Kokiko-Cochran ON, Jiang S, Cardona A, Ransohoff RM, Lamb BT, Bhaskar K. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015 Jun;138(Pt 6):1738-55. Epub 2015 Mar 31 PubMed.
Barrio JR, Small GW, Wong KP, Huang SC, Liu J, Merrill DA, Giza CC, Fitzsimmons RP, Omalu B, Bailes J, Kepe V. In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):E2039-47. Epub 2015 Apr 6 PubMed.
Boston University School of Medicine
This important study extends the authors’ results to show increased TSPO signal in 14 younger and active NFL players in addition to the nine older, former NFL players on whom they previously reported (Coughlin et al., 2015). The study is also in agreement with our postmortem study of a cohort of 66 former American football athletes and 16 non-athlete controls that found increased activated microglial cell density in 18 young football players without CTE (mean age 32 years) and an even greater increase in older former football athletes with neuropathologically verified mild or severe CTE (mean age 44 and 66 years, respectively) (Cherry et al., 2016). TSPO cannot discriminate between microglia and reactive astrocytes; our study showed that activated microglia, not GFAP-positive astrocytes, were increased in football players compared to controls. We also found that the number of years of football play significantly predicted greater density of activated microglial cells in the dorsolateral frontal cortex and that the microglial activation persists for decades after retirement and increases with age. Although Coughlin et al. did not show a significant effect of years of NFL play on TSPO binding, perhaps due to limited sample size, they did not include data on total years of football exposure, which might have proved informative.…More
The authors also did not provide information on the dorsolateral frontal cortex, a region that often shows early pathology in CTE. However, they did show increases in TSPO binding in brain areas particularly vulnerable to tau neurofibrillary degeneration in advanced CTE, namely medial temporal lobe structures (hippocampus, entorhinal cortex, parahippocampal gyri), temporal pole, and supramarginal gyrus, suggesting that inflammation in these regions might accelerate or amplify tau neurofibrillary pathology. They also mentioned that regions such as thalamus and midbrain showed increased TSPO signal, but didn't show these comparisons. Again the thalamus and midbrain are regions of increased susceptibility to tau neurofibrillary pathology in CTE.
Overall, this study adds to the accumulating evidence that microglia activation is an early response to the repetitive head trauma that occurs during football play and can be followed years later by the development of tau pathology and CTE.
It is remains unclear at what point the inflammatory response that is initially protective converts to a self-perpetuating, destructive process that promotes neurodegeneration. Regardless, in vivo measures of glia activation will likely be a good surrogate for detection of brain injury in individuals exposed to repetitive head impacts.
References:
Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen S, Airan R, Kim PK, Adams AV, Garcia C, Higgs C, Sair HI, Sawa A, Smith G, Lyketsos CG, Caffo B, Kassiou M, Guilarte TR, Pomper MG. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol Dis. 2015 Feb;74:58-65. Epub 2014 Nov 7 PubMed.
Cherry JD, Tripodis Y, Alvarez VE, Huber B, Kiernan PT, Daneshvar DH, Mez J, Montenigro PH, Solomon TM, Alosco ML, Stern RA, McKee AC, Stein TD. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2016 Oct 28;4(1):112. PubMed.
Make a Comment
To make a comment you must login or register.