New Role for Parkinson’s Gene DJ-1: DNA and Protein Repair
Quick Links
Too much sugar not only expands your waistline—it can also stir up trouble deep inside cells. As cells age, sugar molecules begin to decorate proteins and DNA building blocks, interfering with their functions. Now it appears the Parkinson’s gene DJ-1 can remedy this. In the June 8 Science, researchers led by Gilbert Richarme at Paris Diderot University report that DJ-1 acts as a DNA deglycase, snipping extra sugars off nucleic acids. In cultured cells lacking DJ-1, DNA accumulates mutations and becomes more prone to breakage, the authors found. The findings complement a previous report by the same group that DJ-1 deglycates proteins. “DJ-1 deglycases may represent the only enzymes that repair both proteins and nucleic acids,” the authors wrote.
Other researchers called this deglycase activity a plausible mechanism for DJ-1’s known protective effects. “This is potentially consistent with the multiple locations where DJ-1 can be found in the cell, including the cytoplasm, mitochondria, and nucleus, and the multiple proposed physiological outputs of DJ-1, which include a very large number of claimed cellular functions,” Mark Cookson at the National Institutes of Health, Bethesda, Maryland, wrote to Alzforum (see full comment below).
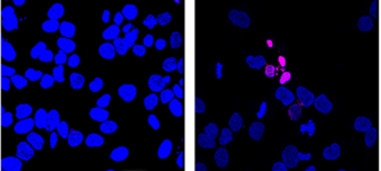
DNA Protector.
Nuclei (blue) of mammalian cells lacking DJ-1 (right) accumulate more broken DNA (purple) than do control cells (left). [Courtesy of Science/AAAS.]
Numerous previous studies had found that DJ-1 helped neurons survive during oxidative stress (see Jun 2004 news; Jul 2005 news; Sep 2005 news). Other research has reported that it acts as a chaperone, preventing aggregation of proteins such as α-synuclein in response to cellular stress (Oct 2004 news). But despite detailed knowledge of DJ-1’s structure, its basic molecular function remained unsolved (Jun 2003 news).
In 2015, Richarme and colleagues first made the case for DJ-1 being a deglycase that restores protein function by removing added sugars from the amino acids cysteine, arginine, and lysine (Richarme et al., 2015). Protein glycation occurs during aging, oxidative stress, and in diseases such as diabetes, and glycation itself boosts the production of reactive oxygen species (Vicente Miranda and Outeiro, 2010).
In the current study, the authors extended their research to nucleic acids. In cell-free solution, DJ-1 prevented the addition of sugars to nucleotides, and also clipped off recently added sugars. In E. coli cultures, bacteria that lacked the DJ-1 homologues had more than twice as much glycated DNA as wild-type cells. Notably, their mutation rate was 46 times higher, indicating a dramatic attack on DNA stability in the absence of functional DJ-1. In the human HeLa cell line, knockdown of DJ-1 led to more glycated and broken DNA as well, again supporting a role in DNA maintenance (see image above).
Does this deglycase activity explain how DJ-1 mutations cause Parkinson’s disease? Tiago Outeiro at Göttingen University Medical Center, Germany, believes it might. He recently reported that glycation of α-synuclein causes the protein to accumulate (Vicente Miranda et al., 2017). In addition, Outeiro had earlier found that DJ-1 directly interacts with α-synuclein to lower its toxicity, and that pathogenic mutations disrupt this interaction, permitting a buildup of toxic α-synuclein through excess glycation (see full comment below).
Richarme’s current paper suggests that faulty DNA repair could also play a role in disease, perhaps by lowering the expression of genes that control proteostasis, Outeiro speculated. “While we cannot dismiss the possibility that DJ-1 performs other functions in the cell, this deglycase activity is extremely exciting, and opens novel avenues for therapeutic intervention,” he wrote to Alzforum (see full comment below).
Cookson suggested that future work should examine whether DJ-1 mutations diminish deglycase activity, and whether glycated proteins and nucleic acids accumulate with age in DJ-1 knockout mice. The authors could not be reached for comment.—Madolyn Bowman Rogers
References
News Citations
- Protection Against Parkinson’s—How the DJ Changes Station
- DJ-1 Dances with Daxx to Keep Neurons Spry
- Drosophila Define DJ-1’s Defensive Role
- DJ Chaperones α-Synuclein—Offers Cell Model of Parkinson Disease
- Spotlight on the DJ: Crystal Structure Solved of Parkinson's Protein
Paper Citations
- Richarme G, Mihoub M, Dairou J, Bui LC, Leger T, Lamouri A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J Biol Chem. 2015 Jan 16;290(3):1885-97. Epub 2014 Nov 21 PubMed.
- Vicente Miranda H, Outeiro TF. The sour side of neurodegenerative disorders: the effects of protein glycation. J Pathol. 2010 May;221(1):13-25. PubMed.
- Vicente Miranda H, Szego ÉM, Oliveira LM, Breda C, Darendelioglu E, de Oliveira RM, Ferreira DG, Gomes MA, Rott R, Oliveira M, Munari F, Enguita FJ, Simões T, Rodrigues EF, Heinrich M, Martins IC, Zamolo I, Riess O, Cordeiro C, Ponces-Freire A, Lashuel HA, Santos NC, Lopes LV, Xiang W, Jovin TM, Penque D, Engelender S, Zweckstetter M, Klucken J, Giorgini F, Quintas A, Outeiro TF. Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain. 2017 Apr 10; PubMed. Correction.
Further Reading
Primary Papers
- Richarme G, Liu C, Mihoub M, Abdallah J, Leger T, Joly N, Liebart JC, Jurkunas UV, Nadal M, Bouloc P, Dairou J, Lamouri A. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science. 2017 Jul 14;357(6347):208-211. Epub 2017 Jun 8 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
National Institute on Aging
The paper by Richarme and colleagues suggests that DJ-1 can repair glycated nucleotides, derived from either RNA or DNA, in addition to the previously claimed removal of glycation products from proteins. Thus, DJ-1 homologues would be the only extant enzymes to repair both proteins and nucleic acids. This is potentially consistent with the multiple locations where DJ-1 can be found in the cell, including the cytoplasm mitochondria and nucleus, and the multiple proposed physiological outputs of DJ-1, which include a very large number of claimed cellular functions.
A couple of questions would be important to follow up on. The first is whether the proposed activity of DJ-1 contributes to DNA or protein repair in the context of an intact mammalian organism. If the authors are correct, and if DJ-1 makes a significant contribution to DNA, RNA, and protein repair in vivo, then one would expect to see accumulation of glycated biomolecules with aging in DJ-1 knockout rats or mice. I mention mammalian contexts because, unlike in lower species, there is only a single DJ-1 homologue in mammals so they might be expected to show greater sensitivity to DJ-1 absence than, for example, bacteria with multiple members of the DJ-1 superfamily. One should be able to measure DNA glycation products or protein glycation products by appropriate mass spectrometry approaches, which would be really interesting.
The second area to be explored is the link to Parkinson’s disease, which wasn’t a specific area of focus in the initial paper but is of interest to many Alzforum readers. Oxidative stress has long been linked to PD, and it is certainly possible that DNA/RNA repair might explain the additional links between DJ-1 and cancer, although there are alternative theories for the latter observations, including the apparent regulation of PTEN activity by DJ-1 (Choi et al., 2014; Kim et al., 2009; Kim et al., 2005).
It would be of interest to know if all the mutations in DJ-1 associated with recessive parkinsonism diminish the measured deglycase activities as predicted for recessive inheritance. Such a link would be further strengthened if other genes for familial PD were also linked to DNA or protein repair, which I don’t think has been suggested to date but might become clear if additional novel mutations are identified.
References:
Choi MS, Nakamura T, Cho SJ, Han X, Holland EA, Qu J, Petsko GA, Yates JR 3rd, Liddington RC, Lipton SA. Transnitrosylation from DJ-1 to PTEN attenuates neuronal cell death in parkinson's disease models. J Neurosci. 2014 Nov 5;34(45):15123-31. PubMed.
Kim YC, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int J Oncol. 2009 Dec;35(6):1331-41. PubMed.
Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu FF, Tsao MS, Mak TW. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005 Mar;7(3):263-73. PubMed.
University Medical Center Goettingen
This study is very interesting, and in line with our own work (Zondler et al., 2014; Miller-Fleming et al., 2014). In particular, I find the deglycase activity extremely relevant, and consistent with our recent work, as we think this process is also associated with the age-related aggregation of α-synuclein (Miranda et al., 2017). That DJ-1 is associated with recessive forms of Parkinsonism fits perfectly with the idea that glycation could constitute a risk factor for PD.
This study now shows that, in addition to the detrimental role we found on α-synuclein, altered DJ-1 function can also be associated with DNA damage. This can form a vicious circle, as it could then affect the expression of genes that might be important to control proteostasis, thereby contributing to disease.
This also suggests, as we pointed out in our studies, that there is an underappreciated connection between conditions such as diabetes and PD, and that this ought to be investigated.
While we cannot dismiss the possibility that DJ-1 performs other functions in the cell, this deglycase activity is extremely exciting, and opens novel avenues for therapeutic intervention.
References:
Zondler L, Miller-Fleming L, Repici M, Gonçalves S, Tenreiro S, Rosado-Ramos R, Betzer C, Straatman KR, Jensen PH, Giorgini F, Outeiro TF. DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson's disease. Cell Death Dis. 2014 Jul 24;5:e1350. PubMed.
Miller-Fleming L, Antas P, Pais TF, Smalley JL, Giorgini F, Outeiro TF. Yeast DJ-1 superfamily members are required for diauxic-shift reprogramming and cell survival in stationary phase. Proc Natl Acad Sci U S A. 2014 May 13;111(19):7012-7. Epub 2014 Apr 2 PubMed.
Vicente Miranda H, Szego ÉM, Oliveira LM, Breda C, Darendelioglu E, de Oliveira RM, Ferreira DG, Gomes MA, Rott R, Oliveira M, Munari F, Enguita FJ, Simões T, Rodrigues EF, Heinrich M, Martins IC, Zamolo I, Riess O, Cordeiro C, Ponces-Freire A, Lashuel HA, Santos NC, Lopes LV, Xiang W, Jovin TM, Penque D, Engelender S, Zweckstetter M, Klucken J, Giorgini F, Quintas A, Outeiro TF. Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain. 2017 Apr 10; PubMed. Correction.
University of Colorado School of Medicine
PARK7/DJ-1 is an amazing gene and protein. Its importance in humans was discovered in 2003 by Bonifati et al., in a family of 12 siblings, three of whom developed early onset Parkinson’s because both copies of their DJ-1 genes were inactivated by mutations (Bonifati et al., 2003).
The gene is ancient and exists in all life forms that use oxygen, including plants. Anaerobic bacteria are among the few creatures that do not have DJ-1. First shown was the protein’s ability to protect cells by controlling oxidative stress and misfolded protein responses.
This paper by Richarme et al. extends the functions of DJ-1 to the repair of glycated DNA. Mammalian and bacterial forms of DJ-1 have similar effects, demonstrating that the structure of DJ-1 molecules has been conserved since the early evolution of oxygen-based life forms.
With the critical importance of DJ-1 in so many biochemical processes, one might think that loss of the gene would be lethal. As shown by Bonifati and colleagues, humans survive to adulthood but do get Parkinson’s. Clearly, other protective mechanisms have co-evolved with DJ-1 to respond to the wide range of stresses that living cells must endure.
References:
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003 Jan 10;299(5604):256-9. Epub 2002 Nov 21 PubMed.
Make a Comment
To make a comment you must login or register.