New Role for Cytokine S100B: Inhibiting Amyloid Aggregation
Quick Links
The cytokine S100B has a reputation as a bad guy in Alzheimer’s disease, revving up inflammation and amyloidosis. Now, new research complicates that view, uncovering a potential beneficial role for the cytokine. In the June 29 Science Advances, researchers led by Cláudio Gomes at the Universidade de Lisboa, Portugal, reported that S100B binds to Aβ42 monomers, oligomers, and fibrils, and hinders their aggregation. This interaction occurs at concentrations of S100B and Aβ42 similar to those seen in the extracellular environment early in AD, implying it could happen in vivo as well, the authors noted. As Aβ continues to accumulate, however, S100B’s proinflammatory effects may take over, swamping its protective effect on aggregation, Gomes suggested to Alzforum.
- The cytokine S100B directly binds to Aβ42 monomers, changing their shape.
- S100B inhibits Aβ42 aggregation, particularly on the surface of amyloid fibrils.
- This inhibitory interaction is stronger in the presence of calcium.
“S100B may link two molecular processes we know are altered in neurodegeneration, protein aggregation and the neuroinflammatory response,” Gomes said. “This is an example of the versatility of biological processes.”
S100B is mostly produced by astrocytes and, at nanomolar concentrations, is known to protect neurons and promote neurite outgrowth. In AD and after neuronal injury, however, its levels surge, pumping inflammation (for review, see Rothermundt et al., 2003). In mouse models of AD, S100B seems to play the villain. Its overexpression exacerbates amyloid deposition and neuroinflammation, while knocking it out lowers plaque numbers and gliosis (Feb 2010 conference news; Craft et al., 2005; Roltsch et al., 2010).
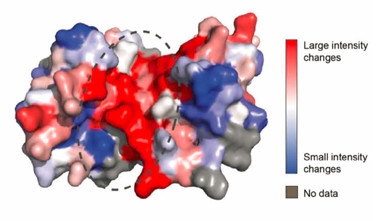
Binding Pocket.
An S100B homodimer containing calcium forms a pocket (dashed circle) where Aβ42 binds. Regions that change shape the most upon Aβ binding are shown in red. [Courtesy of Science/AAAS.]
Gomes previously reported that S100B contains aggregation-prone regions, which are exposed when it binds to calcium or zinc (Fritz et al., 2010; Botelho et al., 2012; Carvalho et al., 2013). For this reason, Gomes wondered if S100B might influence Aβ42 amyloid formation. First author Joana Cristóvão found that S100B interacted with Aβ42 monomers in cell-free solutions, especially when calcium was present. NMR spectroscopy revealed that Aβ42 bound to the center of the S100B homodimer, in a cleft known to recognize several different peptides (see image above). Calcium binding to S100B dimers changes their shape to open up this pocket (Santamaria-Kisiel et al., 2006). Aβ42 in contact with Ca2+-S100B took on an α-helical shape.
Because this shape would interfere with Aβ42’s ability to form β-sheets, the authors looked for an effect on aggregation. Adding S100B to Aβ42 solutions slowed formation of aggregates more than twofold. The amount of inhibition correlated with the concentration of S100B, with 200 μM of the cytokine resulting in a fivefold lower mass of amyloid fibrils forming. This inhibition occurred even without calcium, but the presence of the ion particularly suppressed secondary nucleation events, where Aβ42 oligomers form on the surface of amyloid fibrils. Transmission electron microscopy showed S100B dotting the surface of fibrils, confirming it binds these structures (see image below). Notably, in the presence of S100B, Aβ42 fibrils were simpler than in control solutions, lacking twists and side chains. S100B may act as an extracellular chaperone to suppress amyloid formation, the authors proposed.
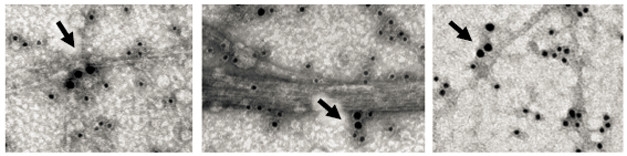
Fibril Inhibition. S100B (large black dots, arrows) binds directly to amyloid fibrils as well as Aβ42 oligomers (small black dots). [Courtesy of Science/AAAS.]
Would inhibiting aggregation help cells? The authors added Aβ42 monomers to the SH-SY5Y neuronal cell line for 72 hours. Cell survival dropped in half, but when Ca2+-S100B was added along with Aβ42, the cells stayed healthy. The finding supports the idea that the cytokine prevents formation of toxic Aβ42 oligomers.
Mathias Jucker at the German Center for Neurodegenerative Diseases in Tübingen said the finding was interesting, but more research would need to show this happens in vivo. Gomes is investigating what other S100 proteins and metal ions do to influence aggregation of Aβ and other neurodegenerative proteins early in disease. He hopes studying these factors could reveal therapeutic targets.—Madolyn Bowman Rogers
References
News Citations
Paper Citations
- Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003 Apr 15;60(6):614-32. PubMed.
- Craft JM, Watterson DM, Marks A, Van Eldik LJ. Enhanced susceptibility of S-100B transgenic mice to neuroinflammation and neuronal dysfunction induced by intracerebroventricular infusion of human beta-amyloid. Glia. 2005 Aug 15;51(3):209-16. PubMed.
- Roltsch E, Holcomb L, Young KA, Marks A, Zimmer DB. PSAPP mice exhibit regionally selective reductions in gliosis and plaque deposition in response to S100B ablation. J Neuroinflammation. 2010;7:78. PubMed.
- Fritz G, Botelho HM, Morozova-Roche LA, Gomes CM. Natural and amyloid self-assembly of S100 proteins: structural basis of functional diversity. FEBS J. 2010 Nov;277(22):4578-90. PubMed.
- Botelho HM, Fritz G, Gomes CM. Analysis of S100 oligomers and amyloids. Methods Mol Biol. 2012;849:373-86. PubMed.
- Carvalho SB, Botelho HM, Leal SS, Cardoso I, Fritz G, Gomes CM. Intrinsically disordered and aggregation prone regions underlie β-aggregation in S100 proteins. PLoS One. 2013;8(10):e76629. Epub 2013 Oct 1 PubMed.
- Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006 Jun 1;396(2):201-14. PubMed.
Further Reading
Primary Papers
- Cristóvão JS, Morris VK, Cardoso I, Leal SS, Martínez J, Botelho HM, Göbl C, David R, Kierdorf K, Alemi M, Madl T, Fritz G, Reif B, Gomes CM. The neuronal S100B protein is a calcium-tuned suppressor of amyloid-β aggregation. Sci Adv. 2018 Jun;4(6):eaaq1702. Epub 2018 Jun 29 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southern California
For the first time, the authors present compelling evidence that S100B is a direct molecular chaperone for Aβ. The upshot is that, at physiological concentrations and with help from Ca2+, a promiscuous S100B motif binds to Aβ monomers and prevents aggregation.
At face value, this may seem to contrast with prior work from our group showing that S100B transgenic-mutant human APP/PS1 mice have exacerbated AD-like pathology. However, as is typical with many protein-protein interactions, binding kinetics are exquisitely concentration-dependent—in this case, stabilizing Aβ monomers at physiological S100B levels but promoting Abβ aggregation at supraphysiological abundance.
Make a Comment
To make a comment you must login or register.