New Reporter Cells Parse Differences Between Tau Strains
Quick Links
Misfolded tau forms fibrillar “seeds” that lure properly folded tau monomers into their toxic fold. Now, using new cell lines designed to detect such molecular subterfuge, researchers led by Nobel laureate Stanley Prusiner at the University of California, San Francisco, report that human tau seeds isolated from different disease tissues have specific requirements for propagation, depending on whether they contain three or four copies of the repeat domain. This paper comes on the heels of a separate study published last month, which also characterized tau strains across a range of different tauopathies (see Kaufman et al., 2016).
In the Prusiner study, human fibrils from four-repeat (4R) or three-repeat (3R) tauopathies were able to corrupt cellular monomers only of the matching isoform. Fibrils derived from diseases, such as Alzheimer’s, in which both 3R and 4R tau isoforms occur side by side in a given fibril, required the presence of both flavors of cellular monomer to kick off propagation. That result suggests that the tau fibrils of AD must be fed by monomers of both isoforms to propagate. However, the findings are far from cut and dried, as ramping up expression of the monomer bait in another cell line overcame some of these apparent strain barriers.
In 2014, researchers at Washington University in St. Louis led by Marc Diamond reported that tau, like prions made of prion protein, can exist in different strains that maintain their unique characteristics when passaged between cells or mice (see May 2014 news). The findings raised the question of whether alternative splice variants of tau, containing either three or four copies of a repeat domain at tau’s C-terminus, might form unique toxic variants that could explain the different pathologies documented across a broad range of tauopathies. For example, 3R tau dominates the Pick’s bodies that characterize Pick’s disease (PiD), while tau aggregates in argyrophilic grain disease (AGD), corticobasal degeneration (CBD), and progressive supranuclear palsy (PSP) consist purely of 4R tau. Neurofibrillary tangles in Alzheimer’s disease (AD) and chronic traumatic encephalopathy (CTE), on the other hand, contain equal parts of 3R and 4R tau.
To better understand the requirements that tau strains from different diseases have for propagation, Diamond, who has since moved his lab to the University of Texas Southwestern Medical Center in Dallas, generated cell lines expressing the repeat domain of 4R tau fused to yellow fluorescent protein (YFP). When seeded with tau filaments that spark aggregation, fluorescent aggregates form in the cells. The researchers used a version of that cell line expressing particularly high levels of 4R tau to reveal that tau within postmortem tissue from AGD, CGD, PiD, and AD patients seeded unique forms of aggregates.
First author Amanda Woerman in Prusiner’s lab and colleagues wanted to use these cell lines to further tease out differences in seeding requirements between the tau isoforms. Analyzing 4R-YFP cell lines via live cell imaging, the researchers found that while tau proteins from the 4R-specific tauopathies AGD, CBD, or PSP kicked off aggregation in the 4R-expressing cells, samples from the 3R tauopathy PiD did not. They next generated a similar cell line expressing the repeat domain of 3R tau, and found the reverse result: PiD samples seeded aggregation, while samples from 4R tauopathies did not. Interestingly, tau proteins extracted from the 3R/4R combo tauopathies AD and CTE did not seed either the 3R- or 4R-expressing cell lines. The researchers next generated another cell line expressing both 3R and 4R repeat domains. This time they found that AD and CTE samples triggered aggregation. This finding suggested that tau seeds from AD and CTE required the presence of both tau isoforms to initiate propagation of a 3R/4R strain of tau.
Previous work with PrP prions had indicated that elevated protein expression boosts the infectivity of prions, and could even overcome seeding barriers between strains. The researchers next tested whether ramping up expression of 4R tau in the cell lines would do the same for tau seeds. They made yet another line with elevated expression of 4R tau, and tested the various brain extracts for infectivity. The 4R tauopathies robustly infected these cells. Surprisingly, two of the six Pick’s disease samples also triggered aggregation, as did samples from AD and CTE.
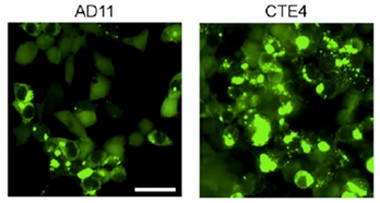
Break the Strain Barrier?
Tau seeds from an AD or CTE patient trigger aggregation in cell lines expressing high levels of 4R tau. [Image courtesy of Woerman et al., PNAS 2016.]
The researchers proposed several possible explanations for how elevated 4R expression would allow seeding from samples thought to contain strictly 3R tau (PiD) or combined 3R and 4R tau aggregates (AD and CTE). One possibility is that the samples actually did contain small amounts of 4R-only aggregates, which were only able to seed cell lines with elevated expression of 4R. In support of this idea, probing two PiD samples that triggered aggregation in these cell lines revealed 4R-tau astrocytic lesions, a condition known as aging-related tau astrogliopathy (ARTAG). These lesions have also been reported in AD and CTE patients, although the researchers did not check for them in those samples.
That AD and CTE seeds triggered aggregation in the 4R-only cell line seemed to contradict the idea that fibrils from these combined tauopathies required both isoforms to be present in the cells to propagate. Another potential explanation is that the elevated expression of 4R tau broke the “strain barrier,” facilitating seeding by the 4R component of combined 3R/4R fibrils. In other words, a new strain arose in the cell lines that was 4R-based. Woerman said the researchers still favor the idea that combined 3R/4R fibrils represent a unique strain. In support of this notion, the tau PET tracer [18F]AV-1451 preferentially binds tau pathology in AD patients, but not in patients with PSP or PiD, suggesting that AD tangles have a unique conformation, Woerman said.
David Sanders of Princeton University, previously a graduate student in Diamond’s lab, is not convinced that tau seeds from AD or CTE require both isoforms to propagate. Without more detailed expression data on the various cell lines, he raised the possibility that the seeding in cell lines expressing both 3R and 4R tau could be explained by their elevated expression of the repeat domains, rather than a necessity for both isoforms. Sanders noted that he and colleagues had also reported that tau fibrils from AD samples could seed aggregation in cells expressing high levels of 4R tau (see Sanders et al., 2014). To Sanders, the real surprise in the new paper was that Pick’s disease samples preferentially seeded cells expressing 3R, rather than 4R, tau. This contradicted previous studies indicating that 3R tau fibrils could seed either 3R or 4R monomers, while 4R fibrils only seeded 4R monomers (see Dinkel et al., 2011). Narrowing down exactly how the repeat domains affect fibril structure and promote propagation will help unlock the code of tau strains, Sanders said.
Prusiner’s study comes on the heels of a recent study from Virginia Lee’s lab at the University of Pennsylvania in Philadelphia, which drew a different conclusion. The study reported that while tau seeds from AD patients contained both 3R and 4R monomers, only one isoform was necessary to facilitate propagation. This suggested tau aggregates in AD could be a mix of exclusively 3R tau, exclusively 4R tau, and 3R/4R fibrils. The findings from both studies underscore the complexity of the unique molecular signature that defines each tauopathy.—Jessica Shugart
References
News Citations
Paper Citations
- Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM, Miller TM, Diamond MI. Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron. 2016 Nov 23;92(4):796-812. Epub 2016 Oct 27 PubMed.
- Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, Barker SJ, Foley AC, Thorpe JR, Serpell LC, Miller TM, Grinberg LT, Seeley WW, Diamond MI. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014 Jun 18;82(6):1271-88. Epub 2014 May 22 PubMed.
- Dinkel PD, Siddiqua A, Huynh H, Shah M, Margittai M. Variations in filament conformation dictate seeding barrier between three- and four-repeat tau. Biochemistry. 2011 May 24;50(20):4330-6. PubMed.
Further Reading
Primary Papers
- Woerman AL, Aoyagi A, Patel S, Kazmi SA, Lobach I, Grinberg LT, McKee AC, Seeley WW, Olson SH, Prusiner SB. Tau prions from Alzheimer's disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):E8187-E8196. Epub 2016 Nov 28 PubMed.
- Guo JL, Narasimhan S, Changolkar L, He Z, Stieber A, Zhang B, Gathagan RJ, Iba M, McBride JD, Trojanowski JQ, Lee VM. Unique pathological tau conformers from Alzheimer's brains transmit tau pathology in nontransgenic mice. J Exp Med. 2016 Nov 14;213(12):2635-2654. Epub 2016 Oct 17 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.