New AD Target: Silencing the NLRP3 Inflammasome with Boron?
Quick Links
Neuroinflammation exacerbates many neurodegenerative diseases, making this process a prime target for therapy. So far, researchers have turned up few specific pathways to modulate, but two new papers now propose hitting the NLRP3 inflammasome. This cytosolic protein machine assembles in response to cellular damage or infection, triggering secretion of pro-inflammatory cytokines that can damage the brain.
- Signaling by the NLRP3 inflammasome exacerbates Alzheimer’s disease.
- Researchers have developed a set of boron compounds that inhibit this signaling.
- Another group found that inhibiting JNK1 kinase also silences signaling.
In the September 1 Cell Chemical Biology, researchers led by David Brough and Sally Freeman at the University of Manchester, U.K., reported development of a set of boron-containing compounds that blocked inflammasome activation through an unknown mechanism. These compounds could form the basis for a new class of drugs, the authors argue. Scientists led by Tao Li and Tao Zhou at the National Center of Biomedical Analysis, Beijing, report in the September 19 Molecular Cell that the kinase JNK1 needs to phosphorylate the protein NLRP3 for it to self-assemble into an active inflammasome. Their findings suggest inhibition of JNK1 as an avenue for therapy. Both approaches are in early investigational phases prior to preclinical testing in AD models.
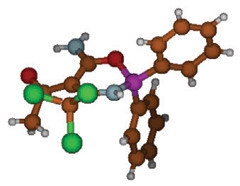
Bring on the Boron.
The inflammatory inhibitor NBC6 contains a boron atom (purple), which gives the molecule its unique shape and properties. [Courtesy of Cell Chemical Biology, Baldwin et al.]
Researchers welcomed the additional options. “There is a lot of interest in inflammasome mechanisms, and several efforts are ongoing in pharma, biotechs, and academia to identify therapeutics that target aspects of the inflammasome activation pathways,” John Davis at the University of Oxford wrote to Alzforum.
NLRP3 inflammasomes first caught the eyes of Alzheimer’s researchers five years ago, when scientists in Germany and the U.S. found that knocking out their signaling in AD mice reduced amyloid accumulation and protected memory (Dec 2012 news). Others reported that Aβ may turn on inflammasome signaling through a scavenger receptor on microglia (Jul 2013 news).
So far, however, researchers have few therapeutic options for dialing down NLRP3 signaling. A small-molecule inhibitor, MCC950, dampens NLRP3 inflammasome signaling in mouse models of multiple sclerosis and other immune diseases, and is under development by researchers led by Luke O’Neill at Trinity College Dublin and Matthew Cooper at the University of Queensland, Brisbane, Australia (Coll et al., 2015). A handful of other compounds have been considered for testing as well, but none have yet caught on (for review see Guo et al., 2015).
To generate more options, Brough and colleagues started with a known NLRP3 inhibitor, the small molecule 2-aminoethoxy diphenylborinate. 2APB is not specific enough for therapeutic use, because it also lowers cellular calcium signaling. First author Alex Baldwin screened a library of 2APB analogs in a cell culture assay for those that suppressed release of the pro-inflammatory cytokine IL-1β. These experiments revealed that the boron atom in 2APB was crucial for its inhibitory effect. The authors then screened a separate library of boron-containing compounds, finding some that were 30 to 60 times more potent than 2APB. They modified these to make them more soluble and effective, developing a series of 31 novel boron compounds (NBCs). NBC6 and NBC13 were particularly potent, more than twice as strong as the unmodified boron compounds, and had good drug properties. Moreover, NBC6 and NBC13 did not affect calcium signaling and were not toxic to a liver cell line.
What does the boron do for these molecules? That is still unclear, Brough said. He noted that boron contains one fewer electron than carbon does, making it more flexible in the types of bond it can form. Boron can link to either three or four other atoms; when it links to three, boron retains a kind of “vacant space” for interacting with other molecules, Brough explained. In the NBC compounds, the ring that contains boron assumes a boat-like shape, with the boron atom sticking up out of the plane, Brough noted (see image above). “The boron in our molecules has some peculiar electron properties that might be key to its effectiveness,” he added.
Boron-containing molecules are typically used for catalyzing reactions, but are uncommon in medicine. One approved cancer drug, bortezomib (Velcade), contains the element. Anacor Pharmaceuticals in Palo Alto, California, develops boron compounds for antimicrobial uses and was recently purchased by Pfizer.
Brough and colleagues have begun testing their NBCs in animals. They injected mice peritoneally with lipopolysaccharide (LPS), which stimulates an inflammatory response through NLRP3, leading to the release of IL-1β. Treatment with NBC13 or the control compound MCC950 prevented IL-1β from rising in inflamed tissues. However, NBC13 was less effective than MCC950 at suppressing plasma IL-1β, cutting levels in half compared with untreated animals but not completely abolishing the response.
Brough said his group continues to refine the NBC compounds to improve their properties. Next up are tests for whether they enter the brain when given systemically. He receives funding from the U.K. Dementia Consortium. The consortium includes several pharma companies interested in developing drugs that hit the NLRP3 inflammasome, and might pick up these molecules, Brough noted.

JNK1 Activates NLRP3.
The kinase phosphorylates the protein, setting the stage for it to self-assemble and begin inflammatory signaling in response to cellular damage. [Courtesy of Molecular Cell, Song et al.]
One factor hampering drug development is that researchers still do not know exactly how the NLRP3 inflammasome activates, although they have found that it requires two sequential inflammatory stimuli as well as deubiquitination of NLRP3 (Bauernfeind et al., 2009; Lopez-Castejon et al., 2013; for review see He et al., 2016). Li and colleagues wondered if phosphorylation of NLRP3 might play a role.
To find out, first author Nan Song mutated each of the six possible phosphorylation sites in human NLRP3 to an inactive analog, then transfected each one into HEK293 cells along with other components of the inflammasome. The only mutant NLRP3 that failed to form an inflammasome was the one lacking the serine 198 residue, and further experiments confirmed that phosphorylation at this site was essential for assembly. Unphosphorylated NLRP3 was unable to form oligomers, and phosphorylation helped strip off ubiquitin tags from NLRP3, suggesting it regulates this process.
Testing various kinase inhibitors, the authors found that only JNK1 inhibitors prevented phosphorylation of S198. They confirmed that JNK1 phosphorylated NLRP3 in cell-free experiments. In addition, JNK1 knockout mice had far less inflammasome activation after LPS stimulation than wild-types did.
While phosphorylation of S198 was required, it was not sufficient for inflammasome assembly, the authors noted. That still required a second inflammatory stimulus. Phosphorylation seems to prime NLRP3 for oligomerization, but not trigger it, the authors hypothesized (see image above). Blocking phosphorylation thus might help prevent inflammasome signaling, they suggested. Brough agreed, adding, “There’s a lot of interest [from pharma] in targeting JNK1.”—Madolyn Bowman Rogers
References
Antibody Citations
News Citations
- Microglia and AD—Does the Inflammasome Drive Aβ Pathology?
- Scavenger Receptor Regulates Inflammasome Activation, IL-1β
Paper Citations
- Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015 Mar;21(3):248-55. Epub 2015 Feb 16 PubMed.
- Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015 Jul;21(7):677-87. Epub 2015 Jun 29 PubMed.
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009 Jul 15;183(2):787-91. Epub 2009 Jul 1 PubMed.
- Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D. Deubiquitinases regulate the activity of caspase-1 and interleukin-1β secretion via assembly of the inflammasome. J Biol Chem. 2013 Jan 25;288(4):2721-33. Epub 2012 Dec 3 PubMed.
- He Y, Hara H, Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016 Dec;41(12):1012-1021. Epub 2016 Sep 23 PubMed.
External Citations
Further Reading
Primary Papers
- Baldwin AG, Rivers-Auty J, Daniels MJ, White CS, Schwalbe CH, Schilling T, Hammadi H, Jaiyong P, Spencer NG, England H, Luheshi NM, Kadirvel M, Lawrence CB, Rothwell NJ, Harte MK, Bryce RA, Allan SM, Eder C, Freeman S, Brough D. Boron-Based Inhibitors of the NLRP3 Inflammasome. Cell Chem Biol. 2017 Nov 16;24(11):1321-1335.e5. Epub 2017 Sep 21 PubMed.
- Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, Han QY, Wang H, Chen Y, Li HY, Li AL, Zhang XM, Zhou T, Li T. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell. 2017 Sep 19; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
The Walter and Eliza Hall Institute
I don’t know how bioavailable the boron compounds will be in the CNS, but the safety profile associated with targeting NLRP3 should be quite good. There are currently five companies that have been formed to make NLRP3 inhibitors, in the hope of getting them into the clinic, although perhaps Alzheimer’s is not the first indication they will target.
Make a Comment
To make a comment you must login or register.