Neurons Derived Directly from Skin Cells Act the Age of Their Donors
Quick Links
Because brain tissues from living donors are not widely available for study, researchers have devised ways to generate neurons from patient skin cells. One problem with this process—which often involves turning fibroblasts into pluripotent stem cells—is that it reprograms neurons to an embryonic state of development. In the October 8 Cell Stem Cell, researchers report that they may have circumvented that issue by directly converting fibroblasts into neurons. And lo and behold, gene-expression profiles of these induced neurons matched the age of their donors. The researchers, led by Fred “Rusty” Gage at the Salk Institute in La Jolla, California, used these neurons to finger RanBP17, a protein that helps keep the nucleus and cytoplasm separate, as a potential master regulator of the aging process. The induced neurons might lead to more age-appropriate cell models of human neurodegenerative disorders, including Alzheimer’s.
“Overall the data argue for the use of induced neurons as a powerful alternative to iPSC derived neurons—in particular for age-related disorders,” commented Lorenz Studer of Memorial Sloan Kettering Cancer Center in New York. “Obviously, it would be interesting to test this hypothesis in patient-specific cells from Alzheimer or Parkinson’s patients.”
Induced pluripotent stem cells (iPSCs) generated from human fibroblasts can be coaxed into a plethora of different cell types, including neurons. Researchers have used them to study genetic and cellular factors that underlie age-related neurodegenerative diseases, including AD (see Mar 2015 news; Jan 2012 news; Aug 2012 conference news). The hope is that neurons derived from patients who carry disease-associated genetic variants will reveal something about disease mechanisms. However, the influence of age, the major risk factor for AD, may be lost when fibroblasts are converted to the embryo-like iPSCs (see Vera and Studer, 2015; Studer et al., 2015). Indeed, researchers have reported that iPSCs from centenarians have the telomere size, mitochondrial metabolism, and gene-expression profiles of embryonic stem cells (see Lappaset et al., 2011). These cells also rapidly divide. Unlike post-mitotic neurons, iPSCs rapidly divide, giving themselves a chance to dispose of defunct organelles and proteins, and repair damaged DNA.
In recent years, using the right mix of transcription factors and small molecules, researchers directly converted fibroblasts into functional neurons, all without a single cell division step (see Jan 2010 news; Pang et al., 2011; and Ladewig et al., 2012).
First author Jerome Mertens and colleagues wanted to produce neurons more representative of the donor’s age. They first set out to determine whether iPSCs were indeed freed from the shackles of aging. They generated fibroblast lines from 19 donors ranging in age from birth to 89 years, and derived iPSCs from 16 of them. They used RNA sequencing to analyze the gene-expression profiles of both the fibroblasts and resulting iPSCs. The researchers found that 78 genes from fibroblasts were differentially expressed between donors younger or older than 40 years of age. However, this age-related genetic signature was virtually wiped out in iPSCs, which only had one differentially expressed gene between older versus younger donors.
The researchers next directly converted the fibroblasts into neurons using established protocols. Strikingly, they found 202 genes differentially expressed between the older (>40) and younger (<40) age groups. Both fibroblasts and iNs had a subset of genes that progressively changed expression with age. Interestingly, these age-related genes in the fibroblasts were almost entirely different to those in the iNs: only seven genes overlapped. This indicated that each cell type had its own aging signature. Indeed, expression of genes related to important skin functions such as wound healing and stress responses changed in old versus young fibroblasts, while for iNs it was genes involved in processes such as calcium homeostasis, neuronal morphology, and synaptic plasticity.

Master Switches of Aging?
Only three genes similarly changed expression with age in fibroblasts, prefrontal cortex, and induced neurons. Researchers propose they regulate aging. [Courtesy of Mertens et al., Cell Stem Cell 2015.]
Age-related expression patterns of only three genes—LAMA3, PCDH10, and RANBP17—overlapped in fibroblasts, iNs, and cortical samples. The researchers reasoned that such common genes could represent master regulators of aging that occurred across cell types. They focused on RanBP17 due to its provocative function. As a member of the importin-β family, RanBP17 forms part of the nuclear pore complex that helps shuttle properly tagged proteins from the cytoplasm into the nucleus. The researchers reported that levels of RanBP17 protein decreased with age in both the iNs and fibroblasts. These findings jibed with a slew of cancer studies that reported RanBP17 among the top age-related genes.
Due to RanBP17’s role as a discerning bouncer for the nucleus, the researchers hypothesized that nucleo-cytoplasmic compartmentalization may falter in aged cells. To measure this, they developed a reporter assay in which they expressed green fluorescent protein (GFP) tagged with a nuclear export signal (NES), and red fluorescent protein (RFP) tagged with a nuclear import signal (NLS). Detection of RFP in the cytoplasm and/or GFP in the nucleus thus served as a handy indicator of a nuclear barrier breach. They found that compartmentalization faltered with age in fibroblasts and iNs, and the severity of the breakdown correlated with dropping levels of RanBP17.
Interestingly, when the researchers used shRNA to dial down RanBP17 expression in fibroblasts from young donors, they found that not only did nuclear leakiness occur, but the cells also started to adopt gene-expression signatures seen in older fibroblasts. This indicated that RanBP17-mediated nuclear compartmentalization served as a master switch for the aging signature.
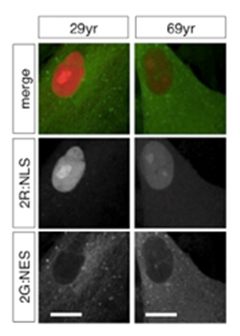
Barrier Breach.
Nuclear proteins (red) and cytoplasmic proteins (green) stay in appropriate compartments in fibroblasts from a young (left) but not old donor (right). [Courtesy of Mertens et al., Cell Stem Cell 2015.]
“These findings raise many exciting questions,” wrote Rick Livesey of the University of Cambridge in England. “Not least, would acute knockdown of RanBP17 in iPSC-derived neurons provide a useful approach to capture some of the biology of cellular aging, and what effects would that have on modeling neurodegeneration in genetic forms of disease?” He added that future studies could compare direct iNs to iPSC-derived neurons taken from patients to understand the relationship between classic aging hallmarks and neurodegeneration.
Slowly dividing or non-dividing cells such as neurons could be particularly vulnerable to an erosion of the nuclear barrier, Mertens told Alzforum. As the cells don’t get a chance to replenish busted nuclear pores during cell division, important nuclear factors may gradually fail to be in the right place at the right time. For example, researchers recently found that expression of the protective transcription factor REST decreases in neurons with age, and that this correlates with cognitive decline (see Mar 2014 news). Mertens wondered whether inappropriate compartmentalization of protective factors such as REST could accelerate neuronal aging.
Importantly, researchers pointed out that while iNs may preserve expression signatures of aging, they would not harbor the scars of aging that neurons accumulate after a lifetime in the brain. Post-mitotic neurons build up DNA damage and possibly a slew of other metabolic, mitochondrial, and epigenetic marks that are specific to the environment of the brain, and those would not necessarily appear in iNs, said Mertens. However, the epigenetic and damage-related aging factors shared by both fibroblasts as well as aging neurons would appear, he added.
Mark Mattson of the National Institute on Aging in Bethesda, Maryland commented that understanding how iPSCs and other stem cells rejuvenate is just as important as studying the retention of aging signatures in iNs. In addition to their expression of developmental factors and their vigorous cell-division cycle, stem cells may also divide in a specific way that helps them clean out the cobwebs of aging. Mattson noted that a recent study suggested that asymmetric cell division in neural stem cells allowed for the disposal of damaged goods in one daughter cell, and the retention of pristine components in the other (see Moore et al., 2015). Learning from these youthful cells could therefore help researchers understand how to combat aging processes in older cells, he said.
Mertens plans to compare aging signatures in iNs from people who are aging well to those from people with familial as well as sporadic Alzheimer’s disease. He proposed that master regulators of aging, such as nuclear compartmentalization, may fail more dramatically in neurons of people with neurodegenerative disease. “It still not quite clear how much of sporadic AD is just severe aging, and how much of it is disease-specific,” he said.—Jessica Shugart
References
News Citations
- Stem Cells Reveal Mechanism Behind Alzheimer’s Risk Factor
- Induced Neurons From AD Patients Hint at Disease Mechanisms
- iPSC Disease Models Up and Coming for AD, Down’s, ALS
- Research Brief: From Fibroblast to Neuron in One Easy Step
- No REST for Weary Neurons: Protective Factor Stems Cognitive Decline
Paper Citations
- Vera E, Studer L. When rejuvenation is a problem: challenges of modeling late-onset neurodegenerative disease. Development. 2015 Sep 15;142(18):3085-9. PubMed.
- Studer L, Vera E, Cornacchia D. Programming and Reprogramming Cellular Age in the Era of Induced Pluripotency. Cell Stem Cell. 2015 Jun 4;16(6):591-600. PubMed.
- Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011 Nov 1;25(21):2248-53. PubMed.
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011 Aug 11;476(7359):220-3. PubMed.
- Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kögler G, Müller FJ, Koch P, Brüstle O. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012 Jun;9(6):575-8. Epub 2012 Apr 8 PubMed.
- Moore DL, Pilz GA, Araúzo-Bravo MJ, Barral Y, Jessberger S. A mechanism for the segregation of age in mammalian neural stem cells. Science. 2015 Sep 18;349(6254):1334-8. PubMed.
Further Reading
Papers
- Rohani L, Johnson AA, Arnold A, Stolzing A. The aging signature: a hallmark of induced pluripotent stem cells?. Aging Cell. 2014 Feb;13(1):2-7. Epub 2013 Nov 21 PubMed.
Primary Papers
- Mertens J, Paquola AC, Ku M, Hatch E, Böhnke L, Ladjevardi S, McGrath S, Campbell B, Lee H, Herdy JR, Gonçalves JT, Toda T, Kim Y, Winkler J, Yao J, Hetzer MW, Gage FH. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015 Oct 6; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.