“Nanosweeper” Brushes Aβ into Cellular Dustbins
Quick Links
Researchers have engineered the tiniest of brooms to tidy up the Aβ-strewn brain. In the May 4 Nature Communications, researchers led by Jing-Ping Zhang of Northeast Normal University in Changchun, China, and Lei Wang and Hao Wang, both of the National Center for Nanoscience and Technology in Beijing, described their generation of “nanosweeper” particles, equipped with both an Aβ-targeting sequence and Beclin-1, a protein known to induce autophagy. The nanosweeper latched onto Aβ and triggered its uptake into cells, where it turned on internal garbage disposals. Injecting the nanosweepers into mice that model AD prevented accumulation of Aβ plaques, and reportedly spared the animals from memory problems. The researchers proposed the nanosweeper as a method to rid the brain of toxic Aβ litter.
- Tiny particles called nanosweepers captured Aβ, triggered its uptake into cells, and switched on autophagy.
- In mice, nanosweepers entered the brain and reduced Aβ burden.
- "Swept" mice had fewer memory problems.
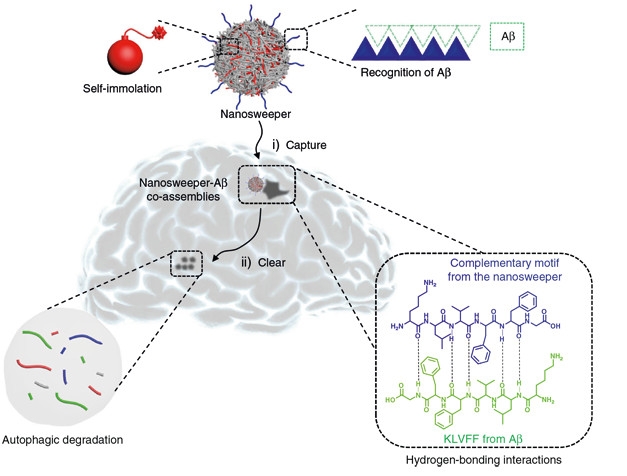
Co-first authors Qiang Luo, Yao-Xin Lin, and Pei-Pei Yang designed the nanosweeper to take advantage of autophagy, the cell’s primary means of disposing of its own detritus. Though Aβ accumulates outside of cells, some cells, such as microglia, engulf the peptide and clear it via autophagy. Beclin-1, which is reduced in the AD brain, activates phagocytosis and autophagy, facilitating the internalization of extracellular debris as well as its digestion (Jun 2010 news; Sep 2013 news). The researchers designed beclin-1-coated nanoparticles to divert extracellular Aβ into this disposal stream.
At their core, the nanoparticles were made of chitosan, a cationic polymer derived from the chitin that gives crustacean shells their toughness. In addition to beclin-1, the researchers coated these particles with the KLVFF peptide, which adheres to Aβ and prevents its aggregation (Xie et al., 2015). In cell culture, these nanosweepers protected mouse neuroblastoma cells from Aβ42 toxicity. While nearly half of the cells succumbed to Aβ42 in the absence of the nanosweeper, nearly all survived in their presence. Both the beclin-1 and KLVFF components were required to achieve maximal protection.
Sweeping Up Aβ. A nanosweeper particle adorned with beclin-1 (red), as well as a complementary peptide (blue) to catch Aβ (green), triggers its own destruction via autophagy. [Courtesy of Luo et al., Nature Communications, 2018.]
The researchers next investigated how the nanosweeper worked. Using biochemical and structural techniques, they confirmed that the KLVFF moiety captured Aβ42 and prevented its aggregation in solution. Immunofluorescence experiments showed that nanosweepers adorned with just the KLVFF peptide triggered uptake of Aβ42 into neuroblastoma cells, but that beclin-1 was required to switch on autophagy. Finally, the researchers observed the internalized nanosweepers trapped inside autophagosomes.
Would this work in the brain? The researchers injected the nanosweepers intravenously into wild-type and APPSwe/PS11de9 mice, along with cyclosporine, a compound known to enhance permeability of the blood-brain barrier. Under these conditions, roughly 2 percent of the injected nanosweepers reached the brain. However, this seemingly small amount was sufficient to trigger autophagy in hippocampal neurons, as evidenced by elevated autophagy markers.

Deliverance—to Destruction? Nanosweepers (red) entered neuroblastoma cells and co-localized with p62 (green), a marker of autophagolysosomes. [Courtesy of Luo et al., Nature Communications, 2018.]
To assess the nanosweepers’ Aβ-clearing ability, the researchers treated nine-month-old AD mice every other day for a month. Compared with control AD mice, treated animals had roughly fivefold less soluble and insoluble Aβ42, and fewer plaques. The treated animals performed at near wild-type levels on the Morris water maze test of spatial memory, a benefit still seen two months after treatment stopped.
“This is a very exciting study that clearly shows that a nanoparticle capable of inducing autophagy and binding Aβ42 can clear amyloid plaques and rescue cognitive capacity,” commented Claudia Almeida of the University of Lisbon in Portugal. However, she was concerned about the fate of the 98 percent of injected nanosweepers that did not enter the brain, wondering if widespread activation of autophagy could have unintended consequences elsewhere in the body.
Almeida also pointed out that although the researchers reported that the nanosweepers bound Aβ42 and activated autophagy, they did not formally demonstrate that the nanoswept Aβ42 was in fact degraded via this autophagic induction. “It could be possible that the acceleration of autophagy could improve neuronal viability independently of Aβ42, which could, in turn, be more capable of eliminating more Aβ42 or reducing Aβ42 production,” she wrote. She also wondered whether the nanosweepers would promote clearance of Aβ by phagocytic cells in the brain, namely microglia and astrocytes.
The researchers did not respond to Alzforum’s request for an interview.—Jessica Shugart
References
News Citations
- Death of the Neatnik: Neurons Perish When Trash Clutters Their Space?
- Poor Digestion in Alzheimer’s Microglia: Is Beclin to Blame?
Research Models Citations
Paper Citations
- Xie H, Qiao Z, Wang H, Duan H, Yang Y, Wang C. Inhibition of β-amyloid peptide self-assembly and cytotoxicity by poly(LVFF-co-β-amino ester). J Pept Sci. 2015 Jul;21(7):608-14. Epub 2015 Apr 30 PubMed.
Further Reading
Primary Papers
- Luo Q, Lin YX, Yang PP, Wang Y, Qi GB, Qiao ZY, Li BN, Zhang K, Zhang JP, Wang L, Wang H. A self-destructive nanosweeper that captures and clears amyloid β-peptides. Nat Commun. 2018 May 4;9(1):1802. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.