Loss of Tau Function Triggers Insulin Resistance in the Brain
Quick Links
Much ado has been made of the neuronal damage inflicted by aggregates of tau, but what about the cost of losing normal tau protein to these proteopathic snarls? In the June 26 Journal of Experimental Medicine, researchers led by David Blum of the University of Lille in France made the case that loss of tau function triggers insulin resistance in the brain. In tau knockout mice, hippocampal neurons responded sluggishly to insulin, laying low synaptic plasticity. Metabolic disturbances extended beyond the brain: The mice overate, were obese, and tolerated glucose poorly. The researchers proposed that loss of normal tau function in early stages of Alzheimer’s could explain the brain insulin resistance observed in some people with the disease. They claim it could even contribute to systemic metabolic disorders—such as Type 2 diabetes—that emerge prior to cognitive symptoms.
“This study is unique and very important, as it offers new data on the physiological functions of tau in the brain insulin response and on metabolism,” wrote Fernanda De Felice of the Federal University of Rio De Janeiro in Brazil.
Tau pathology in the brain correlates with the onset of neurodegeneration and cognitive symptoms in AD as well as other tauopathies. Researchers believe tau wreaks havoc primarily through a toxic gain of function—by forming intracellular aggregates that foul up a host of neuronal functions (see Wang and Mandelkow, 2016). However, tau proteins likely also neglect their normal duties when they join up with these noxious aggregates. Microtubule stabilization and axonal transport are the best-studied functions, but tau has been implicated in a host of other jobs, including controlling neuronal excitability and protecting nucleic acids from oxidative stress (Jul 2010 conference news; Sultan et al., 2011; and Violet et al., 2014). Interestingly, obesity, as well as synaptic and cognitive deficits, have all been documented in tau knockout mice (Kimura et al., 2014; Ma et al., 2014; Ahmed et al., 2015). Given that insulin dysregulation can cause both of these problems, Blum decided to investigate whether tau functions in insulin signaling.
First authors Elodie Marciniak, Antoine Leboucher, Emilie Caron, Tariq Ahmed, and colleagues started by assessing how well hippocampal neurons responded to insulin, using long-term depression (LTD) as a gauge. In hippocampal brain slices from wild-type mice, insulin treatment triggered a nosedive in extracellular field excitatory postsynaptic potentials (fEPSPs). However, in tau KO mice, LTD was much less pronounced in response to insulin. Some evidence of diminished insulin responses was apparent in vivo, when intracerebroventricular insulin injections triggered far less Akt phosphorylation in tau KO than in wild-type animals. Conversely, neuroblastoma cells overexpressing human tau manifested an elevated response to insulin. Together, these findings suggested to the authors that tau plays a role in facilitating neuronal insulin responsiveness.

Tau and Insulin? Tau may promote insulin signaling by blocking the phosphatase PTEN and promoting full activation of IRS-1. [Courtesy of Marciniak et al., JEM 2016.]
How might tau promote insulin signaling? To find out, the researchers dissected the insulin signaling pathway. They found that insulin receptors in tau KO and wild-type mice responded similarly to insulin in that they had similar levels of tyrosine phosphorylation. This was not the case immediately downstream: Tyrosine phosphorylation of IRS-1, which ultimately leads to activation of Akt, was subpar in tau KO mice. Conversely, phosphorylation of several serine residues in IRS-1 was elevated in response to insulin in tau KO neurons. Scientists think phosphorylation of these serine residues occurs via downstream Jun kinases and blocks phosphorylation of tyrosine residues required for optimal IRS-1 signaling. Together, these findings suggested that loss of tau somehow promoted inhibitory phosphorylation of IRS-1. This would stifle its activation and thus reduce the insulin response in neurons.
The researchers found no direct physical interaction between IRS-1 and tau. However, Blum speculated that elevated serine phosphorylation of IRS-1 could be caused by the increased activity of several kinases, including JNK, that has been observed in tau KO mice (Ma et al., 2014). Though tau and IRS-1 did not officially comingle, the researchers noticed tau binding PTEN, a phosphatase that inhibits the PI3K pathway essential for insulin signaling. Tau’s association with PTEN dampened its activity, thus promoting insulin signaling. Together, the findings suggested that tau may influence insulin signaling on two levels: by indirectly influencing the activity of IRS-1, and by direct inhibition of PTEN.
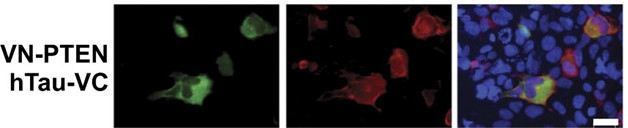
Tau Troubles PTEN. In HEK293 cells, PTEN (green, left panel) and tau (red, middle panel) interact (pink, right panel). Blue is DAPI. [Image courtesy of Marciniak et al., JEM 2016.]
Given tau’s expression beyond the hippocampus, the researchers next asked if its absence might hobble other insulin-dependent events in the brain and beyond. Insulin signaling in the hypothalamus is known to dampen appetite, and wild-type mice largely fasted for a day after an intracerebroventricular insulin injection. Tau KO mice, in contrast, kept munching away. They also gained more weight, had more body fat, elevated levels of circulating leptin, hyperinsulinemia, and glucose intolerance. The researchers attributed these disturbances to an insufficient insulin response in the hypothalamus, although they acknowledged that loss of tau in peripheral organs, such as the pancreas, could also contribute.
Could tau influence insulin signaling in people? An analysis of tau haplotypes suggests as much, the researchers claim. They sifted through 21 genome-wide association studies and found that the H1 haplotype in particular was associated with glucose intolerance. These connections suggested that tau function, which is thought to vary slightly between haplotypes, influences insulin responsiveness and overall metabolism.
“Before this paper, the idea that loss of tau function, rather than gain of toxic function, could be the cause of brain insulin resistance was not on my radar,” commented Dimitrios Kapogiannis of the National Institutes of Health in Bethesda, Maryland. Now he thinks both loss and gain of function could possibly contribute, he told Alzforum.
Studies in animals that develop tau pathology could determine whether loss of functional tau due to aggregation, rather than to genetic knockout, also impairs insulin signaling. Blum proposed that his findings raise the possibility that loss of tau function contributes to the cognitive and metabolic deficits that occur during the disease. Type 2 diabetes is considered a risk factor for AD, and is known to exacerbate Aβ and tau pathology. “We think it could also work in the other direction,” Blum said. “Loss of tau function in the hypothalamus could exert systemic metabolic disturbances.”
Kapogiannis cautioned that more research would need to confirm this claim. Epidemiology could provide support. “If people with AD had a history of weight gain or other symptoms of insulin resistance within the decade prior to the onset of cognitive symptoms, this would support the causal role of loss of tau function,” he told Alzforum.
Kapogiannis is developing a blood biomarker for brain insulin resistance, which samples the contents of brain-derived exosomes (Mullins et al., 2017). He proposed that this type of marker, used in mice that develop tau pathology (such as Li et al., 2016), could help tie tau to metabolism.
“[This study] not only identifies a new function of tau protein as a modulator of brain insulin signaling, but also highlights a potential mechanistic explanation whereby alteration of insulin signaling would occur in AD via tau loss of function,” Maud Gratuze and Emmanuel Planel of the University of Laval in Quebec wrote in an accompanying commentary.—Jessica Shugart
References
News Citations
Paper Citations
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016 Jan;17(1):5-21. Epub 2015 Dec 3 PubMed.
- Sultan A, Nesslany F, Violet M, Bégard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buée L, Galas MC. Nuclear tau, a key player in neuronal DNA protection. J Biol Chem. 2011 Feb 11;286(6):4566-75. PubMed.
- Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Caillierez R, Talahari S, Nesslany F, Lefebvre B, Bonnefoy E, Buée L, Galas MC. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci. 2014;8:84. Epub 2014 Mar 18 PubMed.
- Kimura T, Whitcomb DJ, Jo J, Regan P, Piers T, Heo S, Brown C, Hashikawa T, Murayama M, Seok H, Sotiropoulos I, Kim E, Collingridge GL, Takashima A, Cho K. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014 Jan 5;369(1633):20130144. Print 2014 Jan 5 PubMed.
- Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, Kiosea NC, Nazari S, Chen PP, Nothias F, Chan P, Teng E, Frautschy SA, Cole GM. Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris Water Maze with aging. J Neurosci. 2014 May 21;34(21):7124-36. PubMed.
- Ahmed T, Blum D, Burnouf S, Demeyer D, Buée-Scherrer V, D'Hooge R, Buée L, Balschun D. Rescue of impaired late-phase long-term depression in a tau transgenic mouse model. Neurobiol Aging. 2015 Feb;36(2):730-9. Epub 2014 Sep 28 PubMed.
- Mullins RJ, Mustapic M, Goetzl EJ, Kapogiannis D. Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer's disease. Hum Brain Mapp. 2017 Apr;38(4):1933-1940. Epub 2017 Jan 20 PubMed.
- Li T, Braunstein KE, Zhang J, Lau A, Sibener L, Deeble C, Wong PC. The neuritic plaque facilitates pathological conversion of tau in an Alzheimer's disease mouse model. Nat Commun. 2016 Jul 4;7:12082. PubMed.
External Citations
Further Reading
Papers
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012 Apr;122(4):1316-38. PubMed.
External Links
Primary Papers
- Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A, Dumont J, Issad T, Gerhardt E, Pagesy P, Vileno M, Bournonville C, Hamdane M, Bantubungi K, Lancel S, Demeyer D, Eddarkaoui S, Vallez E, Vieau D, Humez S, Faivre E, Grenier-Boley B, Outeiro TF, Staels B, Amouyel P, Balschun D, Buee L, Blum D. Tau deletion promotes brain insulin resistance. J Exp Med. 2017 Aug 7;214(8):2257-2269. Epub 2017 Jun 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Federal University of Rio de Janeiro
This is an interesting report by Elodie Marciniak, Luc Buee, David Blum, and colleagues providing evidence that tau is necessary for proper brain insulin signaling. Results are significant, as they highlight an as-yet-unexplored physiological role of tau, as well as lead us to speculate whether brain insulin resistance could contribute to tauopathies.
Several reports have now demonstrated that insulin-induced responses are blunted in Alzheimer's disease brains (Ma et al., 2009; Moloney et al., 2010; Bomfim et al., 2012; Talbot et al., 2012). The current report opens new fields of investigation by implicating tau as a key regulator of brain insulin signaling through PTEN phosphatase regulation. It would be important to determine next whether mouse models of tauopathies present brain insulin resistance, and whether it depends on tau phosphorylation, cleavage, or aggregation.…More
Downregulation of brain insulin receptors was shown to trigger memory impairment (Nisticò et al., 2010; Grillo et al., 2015) and tau phosphorylation (Schubert et al., 2004). Although tau regulation of insulin signaling does not appear to directly involve insulin receptors, defective brain insulin signaling could be part of a feed-forward loop driving neuronal malfunction in tauopathies, as now suggested
Finally, the findings that tau regulates non-hippocampal metabolic actions of insulin in the brain is in line with recent evidence underscoring disturbances of peripheral metabolism in AD (Clarke et al., 2015; Crane et al., 2015). Further investigation of tau and insulin intersections would thus indicate whether stimulating brain insulin signaling through repurposed anti-diabetic agents holds potential to attenuate cognitive and metabolic symptoms of tauopathies, as currently under investigation in the AD field.
References:
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Aβ oligomers. J Clin Invest. 2012 Apr 2;122(4):1339-53. PubMed.
Clarke JR, Lyra E Silva NM, Figueiredo CP, Frozza RL, Ledo JH, Beckman D, Katashima CK, Razolli D, Carvalho BM, Frazão R, Silveira MA, Ribeiro FC, Bomfim TR, Neves FS, Klein WL, Medeiros R, LaFerla FM, Carvalheira JB, Saad MJ, Munoz DP, Velloso LA, Ferreira ST, De Felice FG. Alzheimer-associated Aβ oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO Mol Med. 2015 Jan 23;7(2):190-210. PubMed.
Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013 Nov 7;369(19):1863-4. PubMed.
Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015 Nov;64(11):3927-36. Epub 2015 Jul 27 PubMed.
Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009 Jul 15;29(28):9078-89. PubMed.
Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010 Feb;31(2):224-43. PubMed.
Nisticò R, Cavallucci V, Piccinin S, Macrì S, Pignatelli M, Mehdawy B, Blandini F, Laviola G, Lauro D, Mercuri NB, D'Amelio M. Insulin Receptor β-Subunit Haploinsufficiency Impairs Hippocampal Late-Phase LTP and Recognition Memory. Neuromolecular Med. 2012 Jun 3; PubMed.
Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Küstermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Brüning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3100-5. PubMed.
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012 Apr;122(4):1316-38. PubMed.
Make a Comment
To make a comment you must login or register.