Introducing the Coarse-Grained Plaque—A New Type of Amyloid
Quick Links
You’d think that by 2020, pathologists would know all about what amyloid plaques look like? Well, no. Researchers led by Jeroen Hoozemans at Amsterdam University Medical Center have characterized what they believe to be a distinct type. As reported in the September 14 Acta Neuropathologica, these “coarse-grained plaques” can be large and complex structures. At first glance they look similar to the wispy cotton-wool plaques, but they pack tighter and have voids of Aβ in the center. In this regard, coarse-grained plaques also differ from the classic dense-core plaques. Coarse-grained plaques comprise mostly Aβ40, which, in the largest specimen, forms a shell around an Aβ42 core.
- The coarse-grained kind are distinct from other amyloid plaques.
- They abut blood vessels.
- People with early onset AD and ApoE4 have more of them.
Sidling up to blood vessels and packed with norrin, a marker of blood-vessel damage, these plaques are more common in people who carry two copies of the ApoE4 allele or who have early onset Alzheimer’s disease.
“This paper highlights the fact that Alzheimer’s disease is not the same in every case,” said Costantino Iadecola, Weill Cornell Medical College, New York. “It fits with AD being more of a disease spectrum that is influenced by genetics, ApoE status, hypertension, and other vascular risk factors.” Dietmar Thal, KU Leuven, Belgium, agreed. “This study importantly supports the concept of subtypes of AD that differ in their morphological, genetic, and clinical characteristics,” he wrote to Alzforum (see comment below).
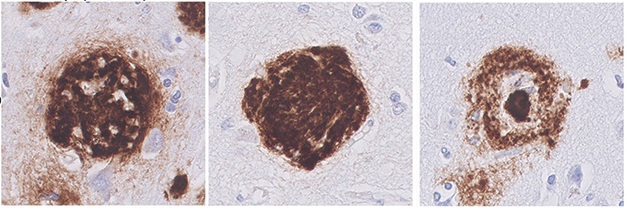
Variation on a Plaque. Coarse-grained plaques (left) lack the clearly delineated borders of the cotton-wool plaques (center) and the compact centers of the dense-core plaques (right). [Courtesy of Boon et al., Acta Neuropathologica 2020.]
Indeed, the authors believe that, like the amyloid deposits found in cerebral amyloid angiopathy (CAA), coarse-grained plaques have a vascular origin. “Whereas CAA occurs when Aβ40 travels across the blood-brain barrier and gets stuck in the blood vessels, coarse-grained plaques may form when Aβ fails to cross the barrier and gets stuck outside the vessels,” first author Baayla Boon told Alzforum.
Hoozemans and colleagues had previously noticed these plaques when comparing pathological hallmarks of typical and atypical AD cases (Boon et al., 2018). “We were flabbergasted that we couldn’t find a name for them and that nobody had documented them as a distinct type of plaque,” said Boon. Two previous papers may have reported a similar type of plaque (Schmidt et al., 1995; Dickson and Vickers, 2001).
Far from peering through a microscope at the Nissl stains of yore, Boon deployed a range of modern techniques to characterize these plaques in 74 postmortem-brain-tissue samples. Thirty-eight of the donors had had early onset AD, 21 had had late-onset, and 15 had no dementia diagnosis but had tested positive for brain amyloid. The researchers used a semi-quantitative analysis to count plaques in each sample, and characterized them with a variety of immunohistochemical techniques, including confocal laser scanning microscopy (CLSM) to visualize plaques in 3D.

Mixed Pathology. Three-dimensional confocal laser scanning microscopy shows Aβ40 (green) and Aβ42 (red) together in a smaller coarse-grained plaque (top), whereas in the larger one, Aβ40 forms a halo. [Courtesy of Boon et al., Acta Neuropathologica, 2020.]
At a diameter of 30-100 μm, these new bullies are bigger than dense-core plaques. In fact, they have multiple cores, pores that are oddly devoid of Aβ, tube-like or trabecular structures running through them, and a vague border, unlike the well-defined rim of dense-core plaques. They stain for APP and prion protein, as do dense-core, and to a lesser extent, cotton-wool plaques, which could indicate damage to adjacent axons. Coarse-grained plaques contain mostly Aβ40, though in the larger ones, this seemed to surround an Aβ42 core (see image above). They are not included in the most commonly used plaque categorization schemes (Thal et al., 2002).
The authors write that the neuroinflammation associated with these plaques is intense. They tested positive for complement 4b, MHC-II, and GFAP, markers of neuroinflammation, microglia, and astrocytes, respectively. The microglia and astrocytes covered and infiltrated the plaques in a pattern that seemed distinct from the other plaque varieties (see image below). Microglia are largely absent from cotton-wool plaques, while they appeared mostly between the core and corona of dense-core plaques. Astrocyte processes were disrupted in coarse-grained plaques, less so in dense-core plaques, and rarely penetrated cotton-wool plaques.

Plaque Panoply. Markers of inflammation (C4b), microglia (MHC-II), and reactive astrocytes (GFAP) suggest unique cellular response to different plaque types. [Courtesy Boon et al., Acta Neuropathological 2020.]
In this small cohort, coarse-grained plaques were absent in cognitively normal cases. Boon found only a single one in one such donor. In contrast, 66 and 95 percent of LOAD and EOAD samples, respectively, had sparse, moderate, or frequent coarse-grained plaques as determined by the semi-quantitative analysis. To these authors, this means that these plaques are particularly relevant to clinical symptoms. What distinguishes people who die with brain amyloid but normal cognition from people who develop dementia is a topic of hot pursuit in the field.
The dense-core plaques associated with ApoE4. Of 28 ApoE4 noncarriers, only 15 had sparse to frequent coarse-grained plaques, whereas 25 of 33 heterozygotes did, and all 11 ApoE homozygous cases scored moderate or frequent. “Taken together, this work highlights once more the importance of the APOE ε4 allele and its specific impact on Aβ pathology, probably in the sense of a separate, APOE ε4-related subtype of AD,” Dietmar Thal, KU Leuven, Belgium, wrote to Alzforum. Thal had previously suggested as much (Thal et al., 2010).
The plaques packed into the sulci of the brain more than its gyri. This might be because sulci are more densely supplied with blood, said Boon, though she added it might also be because brain tissue is more compressed in those anatomical troughs.
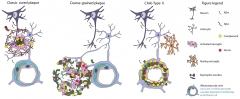
Plaque Origin. The authors believe the coarse-grained plaques are an intermediate between dense-core plaques and CAA based on their content and proximity to blood vessels. [Courtesy of Boon et al., 2020.]
Still, these plaques clearly seem intermingled with the vasculature. In three-dimensional CLSM, 84 percent of 44 scanned plaques directly contacted a blood vessel. Laminin, a component of blood vessel walls, peppered these plaques throughout, whereas it only appeared in the fringes of cotton-wool plaques and the center of dense-core plaques. Another hint of vascular involvement is that they correlated with the presence of CAA type-1 but not CAA type-2—only the former infiltrates capillaries in the cerebral cortex. The authors believe these coarse-grained plaques grow at the border between the blood-brain barrier and the parenchyma.
Iadecola agreed that the vascular connection might be important. “That this Aβ40-related plaque lies close to blood vessels resonates with the fact that ApoE4 reduces blood flow and makes vessels more susceptible to injury,” he told Alzforum. Still, he cautioned that blood vessels run throughout the brain and wondered how often dense-core and cotton-wool plaques contact them by chance.—Tom Fagan
References
Paper Citations
- Boon BD, Hoozemans JJ, Lopuhaä B, Eigenhuis KN, Scheltens P, Kamphorst W, Rozemuller AJ, Bouwman FH. Neuroinflammation is increased in the parietal cortex of atypical Alzheimer's disease. J Neuroinflammation. 2018 May 29;15(1):170. PubMed.
- Schmidt ML, Robinson KA, Lee VM, Trojanowski JQ. Chemical and immunological heterogeneity of fibrillar amyloid in plaques of Alzheimer's disease and Down's syndrome brains revealed by confocal microscopy. Am J Pathol. 1995 Aug;147(2):503-15. PubMed.
- Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer's disease. Neuroscience. 2001;105(1):99-107. PubMed.
- Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002 Jun 25;58(12):1791-800. PubMed.
- Thal DR, Papassotiropoulos A, Saido TC, Griffin WS, Mrak RE, Kölsch H, Del Tredici K, Attems J, Ghebremedhin E. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer's disease. Acta Neuropathol. 2010 Aug;120(2):169-83. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Boon BD, Bulk M, Jonker AJ, Morrema TH, van den Berg E, Popovic M, Walter J, Kumar S, van der Lee SJ, Holstege H, Zhu X, Van Nostrand WE, Natté R, van der Weerd L, Bouwman FH, van de Berg WD, Rozemuller AJ, Hoozemans JJ. The coarse-grained plaque: a divergent Aβ plaque-type in early-onset Alzheimer's disease. Acta Neuropathol. 2020 Dec;140(6):811-830. Epub 2020 Sep 14 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Katholieke Universiteit Leuven, Department of Imaging and Pathology, Laboratory of Neuropathology
Boon et al. describe a novel type of amyloid plaque that is specifically seen in early onset Alzheimer’s disease (AD) cases who have apolipoprotein (APOE) ε4/4 status (Boon et al., 2020). The association of these plaques with capillary cerebral amyloid angiopathy (CAA; CAA type 1) and the APOE ε4 allele is another argument that APOE ε4 carriers may exhibit a type of AD distinct from that of ε4 noncarriers. In this way, this study importantly supports the concept of subtypes of AD that differ in their morphological, genetic, and clinical characteristics.
Earlier work from our group had already indicated cases with capillary CAA that are most frequently APOE ε4 carriers and may represent a distinct type not only of CAA (Thal et al., 2002) but also of AD (Thal et al., 2010). Work by Melissa Murray and colleagues also points to an overrepresentation of the APOE ε4 allele in a specific variant, namely the limbic-predominant variant of AD (Murray et al., 2011). …More
One could argue that the article of Boon et al. just adds one more morphologically distinct plaque type to the concert of many different plaques that all participate in the development of Aβ plaque pathology. However, in my opinion, this point of view would ignore the importance of the genetic link between distinct morphological features and AD. These very specific morphological features seen in AD cases and their association with genetic factors, as seen with the coarse-grained plaques and capillary CAA, teach us about the contribution of the genetic factor, here the APOE ε4 allele, to early onset Aβ deposition and its possible vascular/perivascular clearance, in which ApoE has been shown to play an important role (Deane et al., 2008; Thal et al., 2007). In addition, specific morphological findings help us to distinguish clinical features of AD as shown by Murray et al. (2011) and others (Tomé et al., 2020).
Taken together, the work of Boon et al. highlights once more the importance of the APOE ε4 allele and its specific impact on Aβ pathology, probably in the sense of a separate, APOE ε4-related subtype of AD as previously suggested (Thal et al., 2010).
References:
Boon BD, Bulk M, Jonker AJ, Morrema TH, van den Berg E, Popovic M, Walter J, Kumar S, van der Lee SJ, Holstege H, Zhu X, Van Nostrand WE, Natté R, van der Weerd L, Bouwman FH, van de Berg WD, Rozemuller AJ, Hoozemans JJ. The coarse-grained plaque: a divergent Aβ plaque-type in early-onset Alzheimer's disease. Acta Neuropathol. 2020 Dec;140(6):811-830. Epub 2020 Sep 14 PubMed.
Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008 Nov 13; PubMed.
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011 Sep;10(9):785-96. PubMed.
Thal DR, Ghebremedhin E, Rüb U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002 Mar;61(3):282-93. PubMed.
Thal DR, Larionov S, Abramowski D, Wiederhold KH, Van Dooren T, Yamaguchi H, Haass C, Van Leuven F, Staufenbiel M, Capetillo-Zarate E. Occurrence and co-localization of amyloid beta-protein and apolipoprotein E in perivascular drainage channels of wild-type and APP-transgenic mice. Neurobiol Aging. 2007 Aug;28(8):1221-30. PubMed.
Thal DR, Papassotiropoulos A, Saido TC, Griffin WS, Mrak RE, Kölsch H, Del Tredici K, Attems J, Ghebremedhin E. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer's disease. Acta Neuropathol. 2010 Aug;120(2):169-83. PubMed.
Tomé SO, Vandenberghe R, Ospitalieri S, Van Schoor E, Tousseyn T, Otto M, von Arnim CA, Thal DR. Distinct molecular patterns of TDP-43 pathology in Alzheimer's disease: relationship with clinical phenotypes. Acta Neuropathol Commun. 2020 Apr 29;8(1):61. PubMed.
Make a Comment
To make a comment you must login or register.