Green Glowing Prions Gather in Golgi
Quick Links
Normal cellular prion protein (PrPc) is easy to spot on the surface of neurons and glia in the CNS by antibody staining. But when the protein converts to its toxic scrapie-causing conformation (PrPSc), antibodies no longer recognize it and the aggregates can be very hard to detect. Now, by using an enhanced green fluorescent protein (EGFP)-PrP fusion, Sami Barmada and David Harris at Washington University in St. Louis have gotten the first look at the intracellular location of PrPSc aggregates in brains of mice. Their results, published in the June 15 Journal of Neuroscience, show that the Golgi apparatus fills up with PrPSc long before neurological symptoms appear. After that, PrPSc fills the rest of the neuron, and eventually spills out into extracellular plaques. By allowing direct visualization of pathogenic prions, the PrP-EGFP mice present a new opportunity for tracking the progression of prion disease, and may shed light on the question of where and when scrapie prions exert their neurotoxic effects.
For those studying Alzheimer disease, the Golgi is also of interest as a potential site for amyloid-β peptide production (see ARF related news story). The prion results raise the intriguing question of whether the Golgi could be a breeding ground for protein aggregates in AD or other diseases, as well. (Aβ has been detected in the trans-Golgi network. For example, see Grant et al., 2000).
In previous work, Barmada and Harris generated transgenic mice that expressed the PrP-EGFP under control of the prion protein promoter. They demonstrated that the fusion protein localized normally in neurons and could substitute functionally for cellular PrP in mice with a truncated PrP gene (Barmada et al., 2004). But would the fusion protein allow them to visualize pathological prion production in the mice?
To answer this question, they inoculated the mice intracerebrally with scrapie prion peptides. First, they found that PrP-EGFP cannot produce pathogenic prions on its own, since PrP-EGFP transgenic mice lacking normal endogenous PrP showed no signs of illness. But mice that had both intact PrP and PrP-EGFP did develop neurological symptoms after prion inoculation, though the pathology progressed more slowly than in non-transgenic mice. These results suggested that the fusion protein might be acting as a dominant negative inhibitor of prion propagation. This view was supported by other experiments. The transgene inhibited formation of protease-resistant prion fragments, and PrP-EGFP and PrPSc coimmunoprecipitated, showing that they physically associated in the cell. From these results, Barmada and Harris concluded that PrP-EGFP binds to prion aggregates and inhibits further PrPc recruitment, making the former a useful tag for tracking prion conversion.
And that is just what the authors did, using confocal microcopy to reveal the fine detail of fluorescent signals in brain tissue. They found that the brains of terminally ill scrapie-inoculated mice contained fluorescent aggregates that were not present in healthy, uninoculated animals. The prion aggregates appeared throughout the brain, and were seen in the neuropil as fine granular deposits as well as larger plaque-like spots. Labeling was also observed in neuron cell bodies, assuming a perinuclear distribution. In some parts of the brain, the protein seemed to fill dystrophic neurites and axonal swellings.
In the progression of prion pathology, intracellular fluorescent aggregates appeared before the mice showed any neurological symptoms. At 100 days post-inoculation, no new staining was seen compared to uninoculated mice. But by 150 days, intracellular aggregation of the prion protein was apparent, although the mice would not show any symptoms for another 50 days. By 200 days, intracellular protein plus aggregates in the neuropil were prominent. At the terminal stage (after 202-284 days), most neurons had some accumulation of prion, and extracellular plagues became bigger and more numerous. The changes in distribution were specific for PrP, and were not seen with another membrane-associated protein, Thy1.
Prions Turn Golgi into a Den of Iniquity
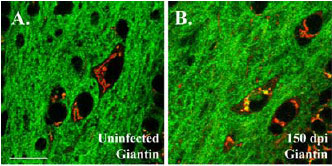
Fluorescent prion aggregates light up the Golgi apparatus in neurons of scrapie-infected mice. Staining of the resident Golgi protein gigantin (red color in uninfected mice) marks where PrP-EGFP gathers (yellow overlay) after inoculation with scrapie prions. The presymptomatic localization of prions to the Golgi presages the later, widespread deposition of both intracellular and extracellular prions. Using PrP-EGFP to get a molecular view of disease progression could give clues to prion pathology, and a way to test therapies. [Image Copyright 2005 by the Society for Neuroscience]
But it was the earliest aggregates, seen at 150 days post-inoculation, which were most informative. These co-localized with the resident Golgi protein gigantin. By the end of the illness, PrP deposits completely filled the Golgi, but did not overlap with ER or lysosome markers. The authors propose that infectious PrPSc gets access to the Golgi via endocytosis, and starts conversion of PrPc there. Alternatively, PrPSc might form in other locations and translocate to the Golgi. Either way, this new method for tracking the travels of PrPSc should be useful not only for watching deposition, but also for assessing effects of therapies aimed at stopping prion disease.—Pat McCaffrey
References
News Citations
Paper Citations
- Grant SM, Ducatenzeiler A, Szyf M, Cuello AC. Abeta immunoreactive material is present in several intracellular compartments in transfected, neuronally differentiated, P19 cells expressing the human amyloid beta-protein precursor. J Alzheimers Dis. 2000 Nov;2(3-4):207-22. PubMed.
- Barmada S, Piccardo P, Yamaguchi K, Ghetti B, Harris DA. GFP-tagged prion protein is correctly localized and functionally active in the brains of transgenic mice. Neurobiol Dis. 2004 Aug;16(3):527-37. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Barmada SJ, Harris DA. Visualization of prion infection in transgenic mice expressing green fluorescent protein-tagged prion protein. J Neurosci. 2005 Jun 15;25(24):5824-32. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.