Girl Power? In Mice, Female Microglia Protect Against Ischemic Injury
Quick Links
Microglia come in male and female versions, and the difference appears to arise early in life, says a new study published June 19 in Cell Reports. Adriana Maggi and colleagues at the University of Milan, Italy, profiled microglia isolated from mice and found they appeared to have distinct identities and functions, determined by sex. In this study, male microglia showed more inflammatory gene expression, while female microglia revealed a more neuroprotective phenotype. Transplanting female microglia into males reduced brain damage after ischemic stroke, while females with male glia showed a trend to more damage. Alzheimer’s and many other neurodegenerative diseases show a sex bias. The results raise the possibility that could be due in part to intrinsic differences in microglial function between men and women.
- Microglia from female mice show a neuroprotective phenotype, male microglia are pro-inflammatory.
- Sexual differentiation appears independent of circulating estrogens.
- Transplanted female microglia reduce brain damage after stroke in males.
As resident immune cells and the brain’s first responders, microglia mop up invaders, dead cells, and other debris. Besides mediating neuroinflammation, they also act as repair crews, manufacturing trophic factors that support neuronal health and stimulate growth of new neurons. The cells also participate in brain development by pruning synapses to shape networks. In Alzheimer’s disease, microglia can help by clearing amyloid, or they can morph into killers—recent studies reveal diverse and dynamic microglial populations in animal models of AD, for better or worse (Jun 2017 news; Sep 2017 news).
Maggi has studied the role of estrogen in microglia action for years. Previously, she was the first to demonstrate the anti-inflammatory actions of estrogen, which shut down microglial activation in response to lipopolysaccharide (Vegeto et al., 2001). To see if sex affected basal microglia function, first author Alessandro Villa analyzed the transcriptomes of purified cells from the brains of 12-week-old male and female mice. RNAseq profiling revealed 204 genes that were more highly expressed in male than female microglia. Many were associated with inflammatory processes, under control of pro-inflammatory transcription factors including nuclear factor κB (NF-κB). The researchers tallied 342 different genes elevated in female microglia, which included many involved in morphogenesis and development, and controlled by anti-inflammatory, pro-tissue-repair factors.
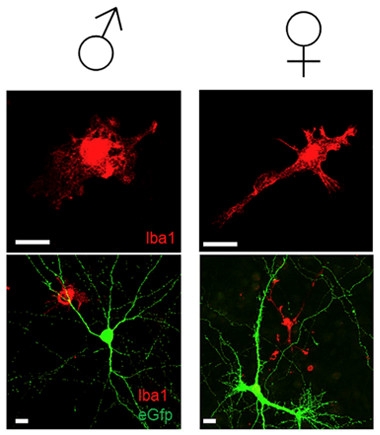
Vive la Différence?
Male microglia (left panels, red) show a pro-inflammatory, globular morphology, while female microglia (right panels) more often display the ramified branches associated with a surveillance function. Lower magnification views (bottom panels) also include neurons (green). [Courtesy of Villa et al., 2018, Cell Reports.]
Other experiments supported the idea that male microglia exist in a state of higher alert than female microglia. In mice engineered to express an NF-κB-responsive version of the luciferase gene, male microglia showed 2.4 times higher luciferase levels than females, indicating ongoing NF-κB activation in vivo. In culture, the cells maintained their sexual identities: More of the isolated male microglia looked activated, with rounder bodies and fewer branching extensions. More of the female microglia resembled resting, ramified cells (see image).
Surprisingly, the female phenotype appeared independent of circulating estrogen. When the researchers ovariectomized females, they did not see a significant shift to a more masculine microglia phenotype, and estrogen replacement did not change gene expression in a predictable way. Even microglia in culture maintained significant sex differences, in the absence of any added hormones. Finally, the authors showed that microglia transplanted from males to females, and vice versa, kept the character of their sex of origin.
How and when does this sex dichotomy originate? In male mice, the brain becomes masculinized shortly after birth, when a surge of testosterone floods the central nervous system. There, it is converted to estrogen and epigenetically modifies the neurons and circuits responsible for male sexual behavior later on. The researchers mimicked this process by giving newborn female mice a shot of estrogen. Once the mice had grown, their microglia showed a more male pattern of gene expression, suggesting that just as neurons respond to masculinizing cues early in life, so do microglia.
Were the male and female microglia functionally different? To find out, the investigators examined the effects of transplanted microglia in a model of acute stroke. After blockage of the middle cerebral artery, male mice develop a larger injury and show higher mortality than females, similar to stroke outcomes seen in men and women. However, when the researchers transplanted microglia from females into males before inducing ischemia, they reduced the lesion area significantly. When they examined the damaged area, the researchers found transplanted female microglia migrating to the lesion. The microglia expressed the anti-inflammatory marker Ym1, suggesting they maintained their protective phenotype to help reduce tissue damage post-stroke. In the converse experiment, male microglia transplanted into females resulted in larger lesions, but the increase was not statistically significant.
“Our results show the female microglia are more protective than male microglia, which is interesting in terms of neurodegenerative disorders,” Maggi told Alzforum. Exactly how that might play out in Alzheimer’s disease is not obvious. For starters, more women than men have Alzheimer’s, and the reasons for that are unclear (for a recent review, see Nebel et al., 2018). Moreover, the idea of protective female microglia does not fit with studies indicating that women show worse symptoms for the same AD pathology as men, Maggi said. Maggi does not know what happens to microglia as mice age, or in the presence of amyloid—she is now looking at older mice to examine microglia over time.
The results are interesting and important, said Stanley Appel, Houston Methodist Neurological Institute in Texas. However, he told Alzforum, sex effects observed in the acute stroke model may not hold up in neurodegenerative diseases. He studies ALS, where younger-onset cases more frequently affect men. “In the stroke model, the investigators looked at an acute neuroinflammatory insult, while neurodegenerative diseases, like ALS, Parkinson’s, and Alzheimer’s, are chronic diseases with continuous exposure of microglia to many environmental influences over many years. And it may be that in that environment, the microglia in both sexes begin to look like each other,” he told Alzforum. In addition, he noted that the investigators did not see sex differences in the transcriptome of peritoneal macrophages, which Appel has reported are also activated in ALS (May 2017 news).
Going forward, it will be important to identify the priming events for inflammatory gene expression in the males, be they epigenetic changes or other mechanisms, and determine how long the effects last, Appel told Alzforum.
The work leaves many questions, which Maggi said she hopes other labs will pursue in a variety of diseases. Her lab is collaborating on studies of ALS and Parkinson’s, both of which show a male preponderance. It will be important to determine if microglial sex differences identified in mice hold up in humans.—Pat McCaffrey
References
News Citations
- Hot DAM: Specific Microglia Engulf Plaques
- ApoE and Trem2 Flip a Microglial Switch in Neurodegenerative Disease
- Inside Out, or Outside In? ALS Turns on Monocytes in Blood
Paper Citations
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001 Mar 15;21(6):1809-18. PubMed.
- Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, Mallampalli MP, Mormino EC, Scott L, Yu WH, Maki PM, Mielke MM. Understanding the impact of sex and gender in Alzheimer's disease: A call to action. Alzheimers Dement. 2018 Sep;14(9):1171-1183. Epub 2018 Jun 12 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, Lolli F, Marcello E, Sironi L, Vegeto E, Maggi A. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018 Jun 19;23(12):3501-3511. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Universitiy Medical Center Hamburg Eppendorf
This paper adds another interesting set of data to the growing list of features attributed to microglia.
The identification of a sex-specific microglia signature in the healthy adult brain should be carefully considered when designing experiments in mice, especially when inflammatory parameters may be involved such as in neurodegenerative or neuroinflammatory diseases. The expression profile of male microglia confer a significantly increased inflammatory predisposition, which was studied in vivo and in vitro in the manuscript. It would be interesting to investigate if this characteristic is also valid in human microglia cells. The sex-specific gene sets that were identified in the study are not directly linked to gene sets that we identified as upregulated in inflammation or neurodegeneration. Therefore, it would be especially interesting to determine the function of the significantly altered sex-specific genes, and if there is a minimal requirement of genes that can switch the sex of microglia in one or the other direction. Alternatively, the sex differences might be determined by epigenetic programming, since the profiles identified seem to be rather cell intrinsic, probably determined very early in life, stable, and not determined by environmental cues. It would be very interesting to investigate how these differences are generated in microglia.…More
A second novelty of the manuscript is the grafting of microglia into the brain via intra-nasal administration. Here, I would have loved to get more data on the basic details of the grafting, for example, immunohistochemistry with microglial homeostasis markers such as TMEM119 or P2ry12. We showed that the latter are quickly downregulated in the diseased brain or after phagocytosis.
I would also be interested to see what happened to the endogenous microglia that usually also start to appear again after CSF-1R inhibition is stopped, and that would easily outnumber the grafted cells. This fact was neglected in the manuscript. Recent investigations showed that depletion of microglia from the brain by CSF-1R inhibition facilitates engraftment of macrophages to the brain (Cronk et al., 2018). Therefore, the phenotype seen by Villa et al. might also be influenced by peripheral immune cells. This should be investigated in more detail in the future.
However, this grafting model might be informative in studying microglia sex differences in neurodegenerative diseases and how their basic expression profiles might influence the phenotype switch toward the neurodegenerative microglia we identified in disease. In SOD1 mice, the mouse model for amyotrophic lateral sclerosis, the microglia homeostatic profile is lost much faster in male mice (Butovsky et al., 2015). We did not perform RNAseq at this time, but only measured microglia-specific genes. It would be interesting to see if and how the basic expression profile of male microglia might contribute to the accelerated disease phenotype. Here, grafting of female microglia into male mice would be very informative.
References:
Cronk JC, Filiano AJ, Louveau A, Marin I, Marsh R, Ji E, Goldman DH, Smirnov I, Geraci N, Acton S, Overall CC, Kipnis J. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med. 2018 Jun 4;215(6):1627-1647. Epub 2018 Apr 11 PubMed.
Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z, Greco DJ, Wu PM, Doykan CE, Kiner O, Lawson RJ, Frosch MP, Pochet N, Fatimy RE, Krichevsky AM, Gygi SP, Lassmann H, Berry J, Cudkowicz ME, Weiner HL. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol. 2015 Jan;77(1):75-99. Epub 2014 Nov 27 PubMed.
Washington University School of Medicine
The paper by Maggi and colleagues shows that microglia acquire a gender bias early on, which is maintained in the adult. A really cool experiment they do is to transplant trans-nasally male or female microglia, and to demonstrate in a model of ischemia that female microglia are more neuroprotective.
The paper is of interest as it could contribute to an explanation of differential susceptibility of men and women to certain neurological diseases.
In the paper, microglia are often identified using markers that have low levels of expression. In future studies, it would be important to use markers that are highly expressed in microglia and are known to distinguish homeostatic versus disease-associated microglia. This is not a major critique, but it is important to corroborate the findings using validated microglia markers that may also have functional significance.…More
University of Milan
Excellent comment. Indeed, this is what we are doing at present time. Our first effort was to evaluate the extent to which the microglial sex difference has a functional significance; now that we have demonstrated this for an acute event, we are interested in continuing in chronic inflammatory diseses such as neurodegenerative disorders.
Make a Comment
To make a comment you must login or register.