G-protein Receptor Patterns Human Cortex
Quick Links
Studying the similarities between humans and mice is easy. Studying the differences can be almost impossible. Take the cerebral cortex. The highly folded, gyrencephalic human cortex has much evolved from the smooth, lysencephalic brain of rodents. So how do you model development of the human cortex? The answer is to try to gain as much information as possible when things go awry.
In today's Science, Christopher A. Walsh at Beth Israel Deaconess Medical Center, Boston, and a host of collaborators from North America, Europe, and the Middle East, reveal that mutations in a G-protein coupled receptor are responsible for an extremely rare, autosomal recessive disorder that is characterized by an excessively wrinkled cortex. Called bilateral frontoparietal polymicrogyria (BFPP), the disorder results in a thinning of the frontal and parietal lobes of the cerebral cortex and numerous small gyri (polymicrogyria). Those affected suffer from cognitive, language, and motor difficulties. The finding sheds light on both the mechanism and evolution of human cortical patterning.
The mutations responsible for BFPP had previously been traced to a 17 centimorgan region of chromosome 16. Lead author Xianhua Piao at the Division of Newborn Medicine, Children's Hospital, Boston, used linkage analysis to narrow this region down to one with only 27 genes, then set about to compare the sequences of these genes with those in normal individuals. Piao found that 22 BFPP patients from 12 different families had mutations in the GPR56. "This is the first time that a G-protein coupled receptor has been implicated in patterning of the human cortex," said Walsh.
Significantly, the mutations (eight in all) are found only in the extracellular domains of the protein. Two splice site mutations near the N-terminus probably result in null mutations, as does a frameshift-causing deletion near the same location. The remaining five are missense mutations. These results strongly indicate that cortical patterning has been compromised by the failure of GPR56 to recognize an extracellular ligand, but the nature of that ligand is unclear at present.
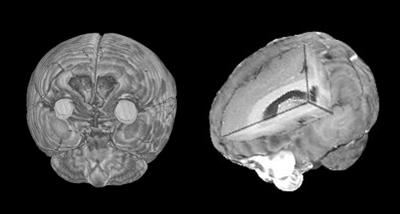
Bilateral frontoparietal polymicrogyria (BFPP) is caused by mutations in a G protein-coupled receptor, reveal Chris Walsh and colleagues in this week's Science. This image shows magnetic resonance imaging (MRI) from a BFPP patient. A mutation in the protein, called GPR56, results in a thinning of the cortex as seen in the cutaway image (right), and a distortion of the cortical folds to yield numerous small gyri (polymicrogyria) as seen in the frontal image (left). Images courtesy of Science.
Neurons are not made in the cerebral cortex. They differentiate from proliferating precursors deep in the brain in the ventricles and subventricular zones. A longstanding theory has suggested that the neurons are guided by a ventricular protomap as they migrate from these zones of proliferation. Like a cruise missile, once they leave the ventricles, their path cannot be altered. A competing theory suggests that migration is not guided by a map and that cortical patterning is determined during or after migration. If the protomap idea is true, then any gene that impacts patterning, as in BFPP, would have to exert its influence before the neurons migrated. To test this theory, Piao investigated exactly where GPR56 is expressed. Using a mouse homolog as a probe, she found that gpr56 mRNA is overwhelmingly expressed in the ventricles and subventricular zones in embryonic animals. In fact, even in adult mice there is some expression in these regions, especially in the dentate gyrus, which has been shown to be a hotbed for neurogenesis in adults. The authors found no expression in the cortex of either embryonic or adult mice, indicating that the protomap theory is correct and the neurons are committed to the cortical pattern before they leave the ventricular zones.
From an evolutionary perspective, the findings are also of some interest. "The Piao et al. study indicates that some genes in the human forebrain may underlie species-specific programs for the generation of cortical neurons that are destined for particular regions of the cerebral cortex," writes Pasko Rakic from Yale University in an accompanying perspective. This is because, though G-protein coupled receptors are well conserved across mammals, the N-terminal end of GPR56 appears to have considerably diverged from mouse to human.—Tom Fagan
References
No Available References
Further Reading
No Available Further Reading
Primary Papers
- Rakic P. Neuroscience. Genetic control of cortical convolutions. Science. 2004 Mar 26;303(5666):1983-4. PubMed.
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, Voit T, Ross ME, Michaud JL, Déscarie JC, Barkovich AJ, Walsh CA. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004 Mar 26;303(5666):2033-6. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.